PCRI Website Clinical Trial Listing_CALIPSO
advertisement

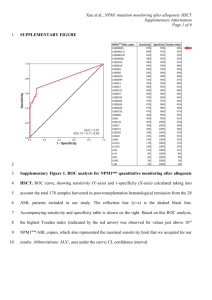
Phoenix Children’s Hospital Research Institute (PCRI) Website Clinical Trial Listing ** Please complete and return to Shy Walker at swalker@phoenixchildrens.com Study Title: A Phase 3 Trial of Calfactant for ALI in Pediatric Leukemia and HSCT Patients Study Purpose: Acute lung injury (ALI) is a common, life-threatening complication among pediatric leukemia and lymphoma and hematopoietic stem cell transplant (HSCT) recipients. Although these children represent a relatively small and unique patient population, they account for the largest proportion of deaths of all pediatric diseases. The long-term goal of this project is to improve outcomes among these patients. Study Summary: Recently, the intratracheal administration of calfactant has resulted in decreased mortality among children with ALI including promising results among children with cancer and following HSCT. Consequently, the primary specific aim of this study is to assess the effect of calfactant on intensive care (PICU) survival among pediatric leukemia and lymphoma and HSCT patients with ALI. Secondary aims include assessment of the effect of calfactant on oxygenation and on the length of mechanical ventilation, PICU stay, and hospital stay. Calfactant therapy has been found to be of benefit in acute lung injury in the overall pediatric population by improving oxygenation and decreasing mortality. These findings, in conjunction with recent subgroup analysis in which calfactant therapy appeared to improve outcomes in immunocompromised children provide the rationale for assessing calfactant therapy in this patient population. Eligibility Criteria: Ages Eligible for Study: 18 Months to 21 Years Genders Eligible for Study: Both Inclusion Criteria: 1. Patients must meet criteria for acute lung injury 2. Patients must have a diagnosis of leukemia/lymphoma undergoing active treatment or following HSCT for any indication. Leukemia/lymphoma will be defined according to the National Cancer Institute Surveillance Epidemiology and End Results Collaborative Staging Manual including those conditions defined as borderline such as myelodysplastic syndromes. All forms of HSCT will be eligible, allogeneic as well as autologous. Exclusion Criteria: 1. Clinical diagnosis of congestive heart failure and/or pulmonary capillary wedge pressure >15 mmHg, or uncorrected congenital heart disease. 2. Glasgow Coma Score < 8 (prior to respiratory failure). 3. Pre-existing limitations on care options, (Do Not Attempt Resuscitation Orders, etc). 4. Patients with impending death from another disease. 5. Patients moribund or with other organ failure at possible randomization Study Location(s): Phoenix Children's Hospital, Children's Hospital of Los Angeles, University of California San Francisco, Riley Children's Hospital, Hackensack University Medical Center, Weill Cornell Medical Center, Maria Fareri Children's Hospital, Rainbow Babies Hospital, Nationwide Children's Hospital, Penn State College of Medicine, Penn State Milton S. Hershey Medical Center, Children's Hospital of Philadelphia, Children's Hospital of Pittsburgh, St. Jude Children's Research Hospital, Texas Children's Hospital, Children's Hospital of Wisconsin Study Contact(s): David Tellez, MD Critical Care Intensivist (602) 933-1784