Paper_Chromatography--Lab_
advertisement

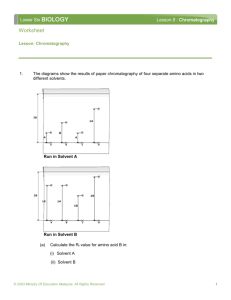
Paper Chromatography Chromatography is a technique used to separate mixtures based on the attraction between the mixture’s components and the stationary and mobile phases. The stationary phase is represented by a piece of paper with the mobile phase consisting of a solvent such as alcohol. The mixture is prepared for chromatography by placing a sample of the mixture onto the stationary phase and then passing a solvent through this sample. Based on the mobile phase, different components of the mixture will separate out of the mixture at different points depending on their attraction to the solvent. Materials: -Chromatography paper -pencils -tape -medicine dropper -3 250ml beakers -aluminum foil -red cabbage -magnolia leaves -solvent (70% isopropyl alcohol) -spinach -mortar & pestle Procedure: 2 trials with each substance 1) Obtain 3 pieces of chromatography paper and handle only the edges of this piece of paper. 2) Place a pencil line ~ 2.0 cm from the bottom of the paper and label a spot for the sample on all 3 pieces of paper. 3) Obtain a piece of spinach and place it into a pestle with 70% isopropyl alcohol. Grind the spinach into a thick liquid using the mortar and pestle. 4) Use a medicine dropper to transfer one drop of your spinach sample to the “labeled” spot on your chromatography paper. 5) Pour approximately 1 ml of solvent into each of the 3 beakers. Do NOT allow the “labeled” spots to become submerged below the solvent level. 6) Immediately close the beaker using the aluminum foil to cover the beaker. 7) Wait approximately 15 minutes or until the solvent line reaches the top of the chromatography paper. 8) With your pencil, make the farthest point reached by the solvent. Known as the “solvent front.” 9) Measure the distance the solvent moved and the distance each mixture component moved. 10) Calculate the Rf value using the following formula. Rf = distance traveled by the pigment from the original location Distance to the solvent front 11) Repeat this same procedure for red cabbage and magnolia leaves. Questions to think about for your conclusion 1) How many pigments were observed in the spinach? Red cabbage? Magnolia leaves? 2) Are these pigments composed of the same substance? If not, what are they? (research this question) 3) Think of 2 improvements or changes you would make to improve this lab. 4) Look at your Rf values for each substance. What can you deduced of the molecular structure/bonding of the substance in relation to the solvent? The differences between the substances in terms of structure/bonding?