Chromatography Lab
advertisement

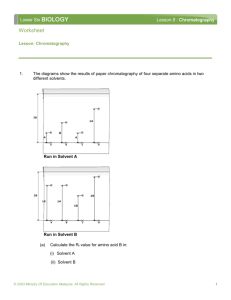
Chromatography Lab Purpose: To determine the number of substances and their Rf values present as dyes in various pen ink. Research: Chromatography is a technique for separating and identifying mixtures of compounds based upon their different rates of adsorption. All types of chromatography employ two different immiscible phases in contact with each other namely the mobile phase and the stationary phase. Normally, the stationary phase is a solid such as paper, starch, alumina, or silica and the mobile phase is a liquid such as water, common organic solvents such as ethanol, or solvent mixtures. The basis for paper chromatography is the fact that porous paper, cellulose, has an enormous surface area to which molecules or ions of substances are attracted (adsorbed) and then released (desorbed) into the solvent as an aqueous solution passes over the paper. Separation of components occur, that is, they will travel at different speeds in a moving solvent because the varying attractions between these components and the paper. This method of separation is known as partitioning. The identity of the components can be deduced by comparing a chromatogram of the unknown mixture with chromatograms of mixtures with known composition (standards). An additional aid in the identification of substances is its Rf value. The Rf value of a compound is a characteristic of the compound and solvent used and serves to identify the constituents of a mixture This can be done by calculation: Rf = Ds / Df Where, Ds = distance traveled by a spot, and Df = distance traveled by the solvent. Hypothesis: Safety: goggles Apparatus/ Materials: Water chromatography paper / filter paper 10cm3 beaker glass cover scissors pencil different color pens. Method / Procedure: 1.) Fill the 10 cm3 beaker with water to about a height of 2 cm. 2.) Use pencil to draw a light line 1cm from one end of the chromatography strip and label as “A” 3.) Draw a second line, 1 cm from line A and label it as “B”. Line A will represent the level to which the strip should be submerged into the solvent, water. Line B will represent the line to which the ink is applied, that is the Line of Origin. 4.) Using the pen, apply a thin ink line over Line B and allow it to dry. 5.) The solvent was allowed to move up the paper. When the solvent front has almost reached the top of the paper, remove it and mark this line with a pencil. 6.) Allow chromatogram to air dry. Chromatography Lab 7.) Measure the distance from line B to each separated dye in the pen ink. 8.) Record the distances measured and save the chromatogram. 9.) Repeat this procedure using the other pens. 10.) Record and tabulate the results of all the pens used. Results: Examples (Use your own data) Data Table: Sample Distance Traveled by component (cm) Distance Traveled by Solvent (cm) Analysis: 1. 2. 3. 4. 5. Define adsorption: Distinguish between adsorption and absorption Explain the stationary phases and mobile phase in chromatography? What causes the components to separate? Source of Error/ Limitations/ Assumptions: Conclusion: Rf Value