VSEPR Model
advertisement

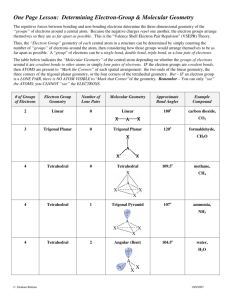
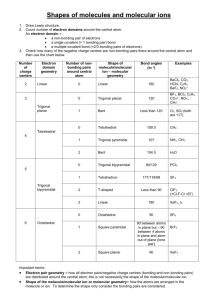
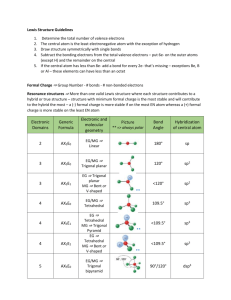
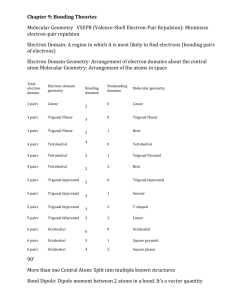
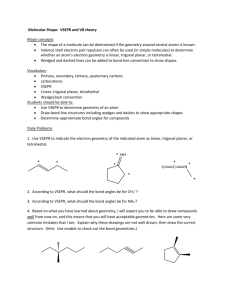
The Valence Shell Electron-Pair Repulsion (VSEPR) model that is based on the repulsive behavior of electron-pairs. This model will show the three dimensional structure of simple molecular (covalent) compounds and polyatomic ions. How to determine molecular geometry (molecular shape) using the table below: 1. Determine the number of valence electrons for all the atoms in a molecule. 2. Add all the electrons for the entire molecule. If the molecule/polyatomic ion has a negative charge, add the number of electrons added. If it has a positive charge, subtract the electrons lost. 3. Determine the arrangement of the molecule, i.e., identify the central atom and the terminal atoms. 4. Distribute all the electrons (by pairs) around the atoms as follows: First: between atoms Second: around terminal atoms (outer atoms) Third: around central atoms 5. Check on stability (8e- around) of each atom. Move electron when needed. 6. When stable electron dot structure is written, count the bonding pairs and non-bonding pairs around the central atom. Note: a. bonding pair of electrons - those electrons shared by the central atom and any atom to which it is bonded. Double bonds and triple bonds are counted as 1. b. non-bonding pairs of electrons - those pairs of electrons on an individual atom that are not shared with another atom. 7. Determine the shape and angle using the table below. Determine the shape and angle of the following molecules: 1. BCl3 2. HCN 3. CO2 4. BF3 5. SO3 6. NO2 7. NH3 8. CH4 9. PCl5 10. SF6 The table below summarizes the molecular and electron-pair geometries for different combinations of bonding groups and nonbonding pairs of electrons on the central atom. # of bonding pair/s of electron on 'central' atom # of lone pair of electrons on 'central' atom 2 3 2 4 0 0 1 0 Electron-pair Geometry linear trigonal planar trigonal planar tetrahedral 3 1 tetrahedral 2 2 tetrahedral 5 0 4 1 3 2 2 3 6 0 trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal octahedral 5 1 octahedral 4 2 octahedral Molecular Bond Geometry Angle linear 180o trigonal planar 120 o bent less than 120 o tetrahedral 109.5 o trigonal less than pyramidal 109.5 o less than bent 109.5 o trigonal 90 o, 120 o and bipyramidal 180 o 90 o, 120 o and seesaw 180 o T-shaped 90 o and 180 o linear 180 o octrahedral square pyramidal square planar 90 o and 180 o 90 o and 180 o 90 o and 180 o Note: for bent molecular geometry when the electron-pair geometry is trigonal planar the bond angle is slightly less than 120 degrees, around 118 degrees. For trigonal pyramidal geometry the bond angle is slightly less than 109.5 degrees, around 107 degrees. For bent molecular geometry when the electron-pair geometry is tetrahedral the bond angle is around 105 degrees. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. http://intro.chem.okstate.edu/1314F00/Lecture/Chapter10/VSEPR.html http://web.gccaz.edu/~ksmith8/VSEPR%20handout.pdf http://www.google.com/search?hl=en&sugexp=les%3B&gs_rn=1&gs_ri=hp&cp=7&gs_id=u&xhr=t&q=vsepr+chart&bav=on.2,or.r_gc.r_pw.r_qf.&bvm=bv.41642 243,d.b2U&biw=1441&bih=654&wrapid=tljp1359506588469012&um=1&ie=UTF8&tbm=isch&source=og&sa=N&tab=wi&ei=iW0IUcfiLJD82gWUroDQBA#imgrc=3VmAuBlIqxrusM%3A%3B6UEnGnaST7D2eM%3Bhttp%253A%252F%252Fwww. molecularmodelscompany.com%252FPortals%252F0%252Fimages%252FVSEPR%252520diagram%252FVSEPR-theorydiagram.jpg%3Bhttp%253A%252F%252Fwww.molecularmodelscompany.com%252FProducts%252FVSEPR%252FVSEPRtheorychart.aspx%3B688%3B491










![VSEPR [Compatibility Mode]](http://s3.studylib.net/store/data/008210566_1-9238cc104b5d8abec6ce7a8d91d0b7ef-300x300.png)



