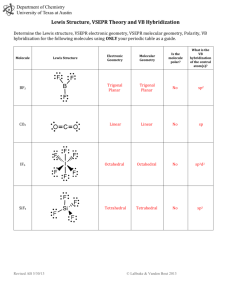
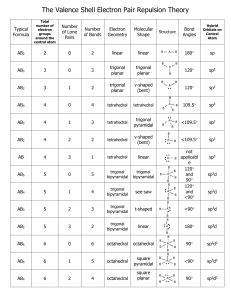
Molecular geom and VBT
advertisement

TURN IN ON MONDAY DEC 2, 2013 TO RECEIVE EXTRA CREDIT 1. For a molecule with the general formula ABn where n=2, the molecular shape may be ________. a. linear or bent b. maybe linear or trigonal planar c. is T-shaped d. is trigonal planar 2. According to the VSEPR theory, if there are 5 pairs of electrons in the valence shell of an atom, they will be arranged in a(n)____________________ geometry. a. octahedral b. linear c. tetrahedral d. trigonal planar e. trigonal bipyramidal 3. The molecular geometry of _____ is square planar. a. CCl4 b. XeF4 c. PH3 d. XeF2 e. ICl3 5. The F-Cl-F bond angle in ClF3 is ______________. a. 109.50 b. 1200 c. 1800 d. 900 e. slightly less than 109.50 6.The molecular geometry of PHCl2 molecule is _____________. a. bent b. trigonal planar c. trigonal pyramidal d. tetrahedral e. T-shaped 7. The F-Xe-F bond angle in the XeF4 molecule is about _________. a. 900 b. 109.50 c. 1200 d. 1800 e. 600 8. The central iodine atom in IF5 has _____ unbonded electron pairs and ____ bonded electron pairs in its valence shell. a. 1,5 b. 0,5 c. 5,1 d. 4,1 e. 1,4 9. a. b. c. d. e. According to the VBT, which orbitals overlap in the formation of the bond in HBr? 1s on H and 4p on Br 1s on H and 4s on br 1s on H and 3p on Br 2s on H and 4p on Br 2s on H and 3p on Br 10. The electron-domain geometry of a carbon-centered compound is tetrahedral. The hybridization of the central carbon atom is _______. a. sp b. sp2 c. sp3 d. sp3d e. sp3d2 11. Generally, a molecule in which the central atom is sp3d2 hybridization only in ______. a. PCl5 b. XeF4 c. PH3 d. Br3 e. BeF2 12. The hybridization s of bromine in BrF5 and of arsenic in AsF5 are _____ and ____ respectively. a. sp3, and sp3d b. sp3d and sp3d2 c. sp3d and sp3 d. sp3d2 and sp3d2