File
advertisement

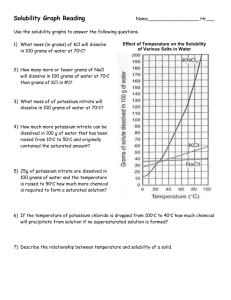
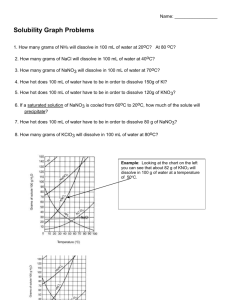
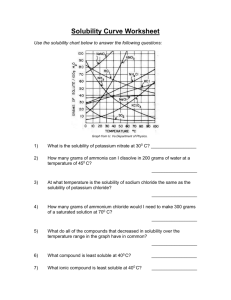
8.5 Solubility and Saturation The Degrees of Saturation: Solubility is the quantity of that can dissolve in a given quantity of . Quantitatively, solubility is expressed as the mass of solute that can be dissolved per g of at a given temperature. Define the following terms: Saturated Solution: Unsaturated Solution: Supersaturated Solution: Solubility Curves Y-axis: Solubility of substance (g/100 g H2O) Substances: Compound being dissolved in water (H2O) X-axis: Temperature (Celsius) How does the solubility of sucrose (table sugar) change as the temperature increases? What is a solubility curve? Intepereting a Solubility Curve Each point on the solubility curve shows how many grams can be dissolved at a specific temperature. Each line shows how much substance can dissolve as a function of the temperature of the solution. Example: Answer the following questions: How many grams of potassium chloride (KCl) can dissolve in 100 grams of water at 20°C? How many grams of potassium nitrate (KNO3) can dissolve in 100 g of water at 40°C? How many grams of sodium chloride (NaCl) can dissolve in 100 g of water at 100°C? How many grams of potassium chlorate (KClO3) can dissolve in 200 g of water at 80°C? At what temperature can 75 grams of potassium nitrate (KNO3) dissolve in 100 g of water? At what temperature can 30 grams of potassium chloride (KCl) dissolve in 100 g of water? How we numerically describe saturation: Saturated: solute = solubility Unsaturated: solute < solubility Supersaturated: solute > solubility Sample Problem (see p.395): A potassium sulfate solution, K2SO4 (aq), containing 11.8 g/100 g H2O at 20 °C, is warmed to 60°C. What additional mass of potassium sulfate is required to saturate this solution at 60°C? Solubility of Gasses What do you notice about the solubility of oxygen as temperature increases? Homework: p. 395 Practice #1-3 p. 137 #1-4, 6