Water C/D Learning Targets - Fort Thomas Independent Schools
advertisement

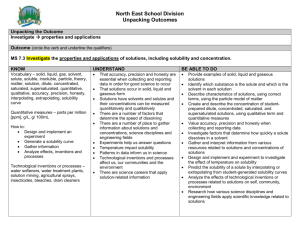
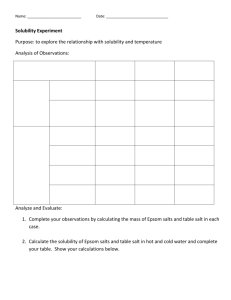
Unit 1 Water C/D Learning Targets 1. I can name ionic compounds when given the formula. (pgs. 38-40 ion reference sheet) 2. I can write the formula when given the chemical name. (pgs. 38-40 ion reference sheet) 3. I can explain solubility in terms of a solute and a solvent. (pg. 29, 53) 4. I can define the terms: saturated, unsaturated, and supersaturated (pg.53) 5. I can correctly interpret a solubility curve (pg. 54) to determine solubility as a function of temperature (pg. 55 C.2 Dev. Skills, Worksheet C.1, Lab C.3 pg. 57) 6. I can classify a solution as saturated, unsaturated, or supersaturated when given a set of conditions and solubility curve (graph) (C.5 pg. 62 Modeling Matter Activity) 7. I can design a procedure to test the solubility of a substance in water at various temperatures. (C.3 Lab) I can identify the control, the variable being tested and explain the data collected? 8. I can draw and explain how the polar nature of water enables it to dissolve ionic substances. (pg. 59-61, fig. 1.39 pg. 61) 9. I can solve problems relating to solution concentration in percent’s, ppm's, and ppb's. (pg 63-64 C.7 Developing Skills, Worksheet C.6) 10. I can describe the difference between heavy metals and essential metals. (pg. 65-67) 11. I can list and describe three examples of essential metals and two examples of heavy metals. (pg. 65) (group pp) 12. I can list some of the adverse health effects of heavy metals on humans. (group pp) 13. I can list some sources of lead and mercury contamination found in natural bodies of water. (group pp) 14. I can classify a solution as acidic, neutral, or basic given its pH value.(pg. 70) 15. I can recall the EPA's acceptable pH range for drinking water and fish. (pg. 70) 16. I can explain the differences between acids and bases based on the ions that are present. (pg. 68) 17. I can recognize formulas for substances as either being an acid or a base. (pg. 69) 18. I can explain how molecular substances are different from ionic substances. (notes) 19. I can classify a solute as being polar or non-polar based upon its relative solubility in various solvents (water, ethanol, lamp oil). (C.11 Solvents Lab pg.72-75) 20. I can explain how temperature and pressure affect the solubility of a gas in water. (pg. 75-77) 21. I can explain how fish can suffocate in water where the temperature has increased for several days. (pg. 77) 22. I can explain how power plants and other industries contribute to increase temperature in surrounding water bodies. (pg. 78) 23. I can explain Gas Bubble Trauma. (pg. 78) 24. I can analyze a given set of data and use graphing to determine if a parameter is "normal" or "abnormal" compared to a historical set of data. (Class Activity C.14) 25. I can list the three purification processes in the earth's hydrologic cycle and explain the types of materials removed by each process. 26. I can explain the purpose of the following processes in municipal water treatment: screening, prechlorination, flocculation, settling, sand filtration, postchlorination, aeration, and fluoridation. (pg 88) 27. I can compare and contrast the hydrologic cycle and the Northern Kentucky Municipal water treatment system. (pg. 89 D.3) 28. I can list the advantages and risks associated with chlorinating water. I can discuss possible ways to eliminate chlorine and their disadvantages. (pg. 91) 29. I can define hard water and know which elements are responsible for hardness (pg. 92) 30. I can explain how soap's cleaning power is affected by water hardness. (pg. 96) 31. I can describe how a resin-based water softener works. (pg. 99) 32. I can discuss the world water crisis in terms of where and why. I can also describe some impacts and the future of water. (group pp) 33. I can describe several chemical contaminants of water, where they come from and the impact they have. (group pp) 34. I can describe several biological contaminants, where they come from and the impact they have. (group pp) 35. I can use the following terms with respect to water purification techniques (sustainable, flocculation, coagulation, filtration, activated charcoal, disinfection, chlorination, SODIS, PUR™) (group pp)