Chemistry Name: Solutions Quiz Date: Multiple Choice: Choose the
advertisement

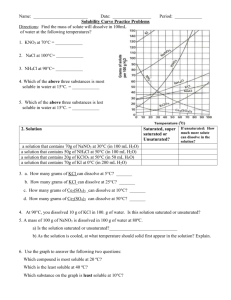
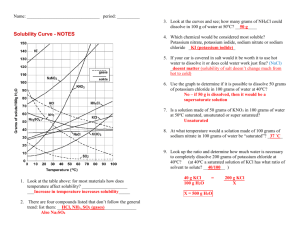
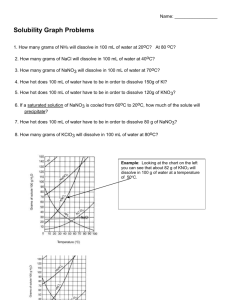
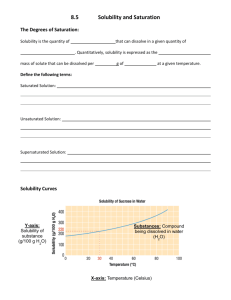
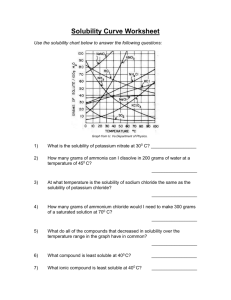
Chemistry Name:________________________ Solutions Quiz Date:__________________ Multiple Choice: Choose the best answer and place the letter in the blank provided. ____1. a. Pure water will conduct an electric current true b. false ____2. a. Supersaturated solutions are extremely unstable true b. false ____3. a. Solutions are only solids dissolved in water true b. false ____4. a. Ionic salts dissolve well in water because of water’s polarity b. viscosity c. boiling point d. freezing point ____5. a. Which of the following is not a solution? air b. bronze c. 70% isopropyl alcohol d. soil ____6. a. Which of the following solutions would be an electrolyte? KI b. NaCl c. C12H22O11 d. a and b ____7. a. Why are molecular compounds non-electrolytes? they never dissolve in water b. they dissociate into ions when they dissolve c. they dissolve as a whole molecule d. there is a movement of ions when they dissolve ____8. In the following solubility curve, where (a, b or c) would the substance be unsaturated? ____9. a. Why is water a polar molecule? O is more electronegative than H b. H is more electronegative than O c. O and H have the same electronegativity value d. O and H share the electrons in the bond equally Given the water molecule below, answer questions 10-13. 10. Which end of the water molecule (a or b) would be partially positive (δ+)? ____ 11. Which end of the water molecule (a or b) would be partially negative (δ-)? ____ 12. Which end of the water (a or b) would the cation of the solute be attracted to? ____ 13. Which end of the water (a or b) would the anion of the solute be attracted to? ____ Use the solubility curve below to answer questions 14-19. 14. What is the mass of KCl that is soluble at 80 °C? _____ 15. What is the mass of KClO3 that is soluble at 30 °C? _____ 16. What is the most soluble substance at 10 °C? _____ 17. At what temperature would exactly 80 grams of KNO3 be soluble? _____ 18. Name a substance in which solubility does not increase as temperature increases?_____ 19. List a temperature at which 100 g of NaNO3 would be supersaturated. _____ Short Answer: Answer the following questions completely and concisely. 20. Why does the solubility of most salts increase as temperature increases? (2 pts) 21. Name two of the three ways that dissolution can be sped up and explain WHY (4 pts). Problems: Solve the following problems showing all work for full credit. 22. How many grams of MnO2 are needed to make 5.6 liters of a 2.1 M solution? 23. What is the concentration (molarity) of a solution with a volume of 9.00 mL that contains 2.00 grams of Fe(OH)3? 24. How many liters of 3.4 M solution can be made using 78 grams of isopropanol (C3H8O)? 25. If I dilute 250. mL of 0.10 M lithium acetate solution to a volume of 750. mL, what will the concentration of this solution be? (MCVC = MDVD)