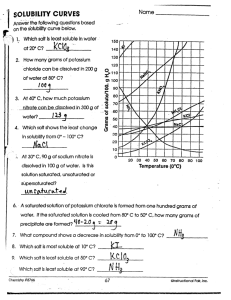
Solubility Graph Reading
advertisement
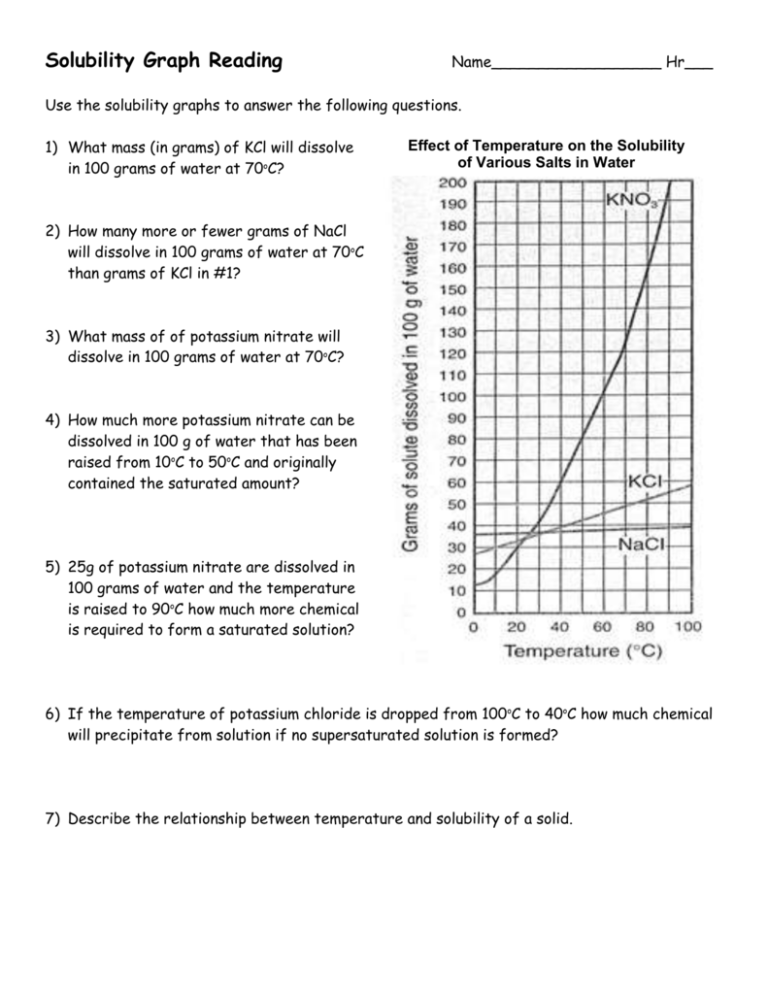
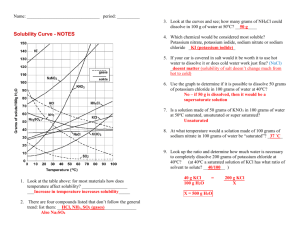
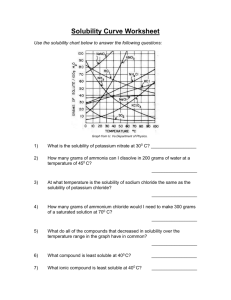
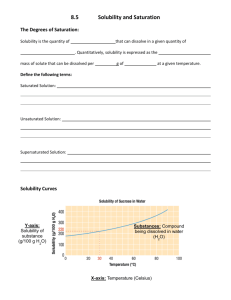
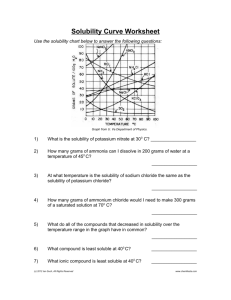
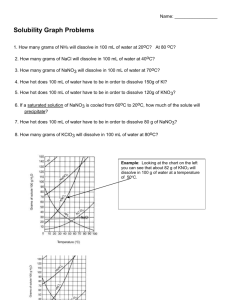
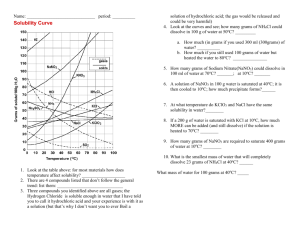
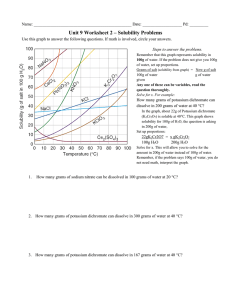
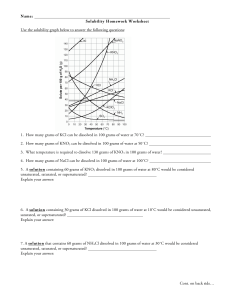
Solubility Graph Reading Name__________________ Hr___ Use the solubility graphs to answer the following questions. 1) What mass (in grams) of KCl will dissolve in 100 grams of water at 70oC? Effect of Temperature on the Solubility of Various Salts in Water 2) How many more or fewer grams of NaCl will dissolve in 100 grams of water at 70oC than grams of KCl in #1? 3) What mass of of potassium nitrate will dissolve in 100 grams of water at 70oC? 4) How much more potassium nitrate can be dissolved in 100 g of water that has been raised from 10oC to 50oC and originally contained the saturated amount? 5) 25g of potassium nitrate are dissolved in 100 grams of water and the temperature is raised to 90oC how much more chemical is required to form a saturated solution? 6) If the temperature of potassium chloride is dropped from 100oC to 40oC how much chemical will precipitate from solution if no supersaturated solution is formed? 7) Describe the relationship between temperature and solubility of a solid. 8) What mass of oxygen can be dissolved in 100 grams of water at 15 oC? Effect of Temperature on Solubility of Gas Molecules in Water 9) What mass of nitrogen monoxide can be dissolved in 100 grams of water at 30 oC? 10) How much more soluble in water is carbon monoxide than nitrogen gas at 10oC? At 40oC? 11) A. If a glass of soda is left outside on a hot summer day, what would you expect to happen to the amount of dissolved carbon dioxide. B. Why do you suppose some people prefer to drink carbonated beverages at very cold temperatures? 12) Describe the relationship between temperature and solubility of a gas.