Reading Guide - Chemistry Courses: About
advertisement

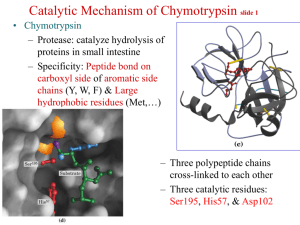
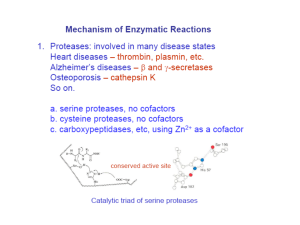
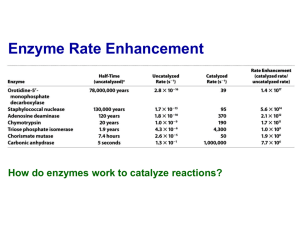
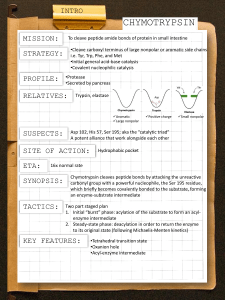
Reading Guide: Pratt and Cornely, Chapter 6 1. What are the advantages and disadvantages of stability of chemical bonds such as the peptide bond? 2. What are the three general ways to increase the rate of a reaction? 3. What is a catalyst? How are enzymes superior to chemical catalysts? 4. Enzymes can be named according to a descriptive nomenclature—how is this advantageous and disadvantageous. List the 6 classes of enzymes according to the EC nomenclature. 5. Draw an energy diagram for a one-step reaction that is spontaneous and slow. Label the figure and axes. Explain how a reaction can be both spontaneous and slow. 6. Using the rule of thumb provided, how much must a transition state be lowered in energy to produce a 1000x increase in rate? 7. Define cofactor, coenzyme, and cosubstrate. 8. The transition states in the figures on p165 are wrong. Explain. 9. List three types of chemical catalysis. Which sidechains can be involved in each type? 10 What is the catalytic triad of chymotrypsin? What is the function of each residue? Draw an organic mechanism of the catalyzed reaction of hydrolysis of an amide bond. 11. How was DIP used to help elucidate the mechanism of chymotrypsin? 12. If an enzyme fit the substrate like a lock and key, it would slow the reaction down. What did Linus Pauling suggest about the lock and key model of enzyme binding in 1946? 13. What is the chemical basis of chymotrypsin’s ability to stabilize the transition state of peptide hydrolysis? Which amino acids are involved? 14. Define proximity effect, orientation effect, induced fit, and electrostatic catalysis. 15. How does induced fit help hexokinase to avoid wasting energy? 16. How do enzymes with similar catalytic strategies exhibit different substrate specificity? 17. Define zymogen. How is a protease essential for blood clotting? How does heparin work as an anticoagulant drug?