Supplementary Methods - Word file (29 KB )
advertisement

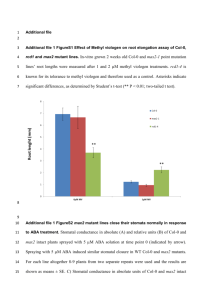
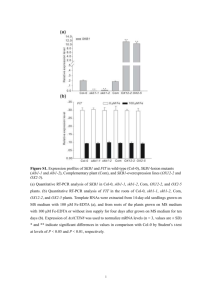
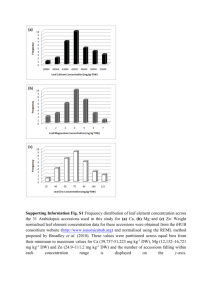
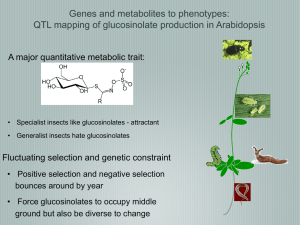
Supplementary Methods QTL mapping with near-isogenic lines. We grew the plant material (our original recombinant near-isogenic lines) and determined dry weight as described1. In general, we performed 13 replicates á 5 plants per line. For each replicate, we obtained mean dry weight per plant by dividing the total dry weight for all plants in a given replicate by the number of plants in the replicate. Afterwards, we used ANOVA to obtain least square means (LSM) for each line according to the following model: MEAN DRY WEIGHT = CONSTANT + LINE + REPLICATE. Finally, we tested for the association between markers within the 210-kb interval and LSMDRY WEIGHT using single marker analysis and F-ratio statistics. The data set includes near-isogenic lines that harbor recombination breakpoints in the 210-kb interval investigated in this study. For several of these lines, we obtained F2 progeny from the original Col-0 x CL5 cross heterozygous for the GS-AOP2 glucosinolate biosynthesis locus on chromosome 4. In subsequent generations, we generated progeny homozygous Col-0 or Ler0 at this GS-AOP locus. We included both progeny (designated ‘a’ or ‘b’) in the initial analysis for growth rate QTL shown in Figure 1. Likewise, we included two lines, J19 and J25, in our analysis that we initially scored recombinant3 but, with the use of more markers, later turned out to be non-recombinant. Columns indicate lines, LSMDRY WEIGHT, followed by genotypes (C: Col-0; L: Ler-0) at GS-AOP, and within the investigated 210-kb interval; numbers refer to marker positions. Genotyping. We extracted DNA from freeze-dried tissue according to3. PCR primer pairs used for genotyping are listed in Supplementary Table 1. We sequenced PCR products either directly (grLC1, 3, 5, and grCL6), or separated them on 4% MetaPhor (BMA, Rockland, ME, USA) agarose (glLC, glCL, grLC2, grCL6, grCL7), or, in case of grLC4 and grCL9, on 1.5% SeaKem LE (Cambrex Bio Science Rockland, Inc., Rockland, ME, USA) agarose gels. For all but one experiment, observed genotype frequencies did not deviate significantly from the expected 1:2:1 ratio for homozygous Col-0, heterozygous, and homozygous Ler-0 genotypes at the segregating intervals. Only for the experiment involving a mixture of grCL7 and grLC2 progeny, we observed too few plants with a Ler-0 background 5’ and a Col-0 background 3’ of the ‘downstream’ growth rate QTL when the genotype at this growth rate QTL was Col-0. Sequence survey. We amplified a ~ 6.4 kb DNA portion containing At5g23160 and At5g23170 from 31 Arabidopsis accessions randomly selected from the species’ natural range: Aa-0 (Aua/Rhön, Germany), Ag-0 (Argentat, France), Bl-0 (Bologna, Italy), Bla-10 (Blanes/Gerona, Spain), Cal-0 (Calver, U.K.), Can-0 (Canary Islands, Spain), Col-0 (Columbia), Cnt-1 (Canterbury, UK), Cvi-0 (Cape Verdi Islands), Di-0 (Dijon, France), Di-1 (Dijon, France), Di-g (Dijon, France), Ema-1 (East Malling, U.K.), Ka-0 (Kaernten, Austria), Kas-1 (Kashmir, India), Ler-0 (Landsberg erecta), Lip-0 (Lipowiec/Chrzanow, Poland), Ma-0 (Marburg, Germany), Mt-0 (Martuba/Cyrenaika, Libya), No-0 (Nossen, Germany), Oy-0 (Oystese, Norway), Pa-1 (Palermo, Italy), Per-1 (Perm, Russia), Petergof (Petergof, Russia), Pi-0 (Pitztal/Tirol, Austria), Sei-0 (Seis am Schlern, Italy), Sorbo (Tadjikistan), Su-0 (Southport, UK), Tac (Tacoma, Washington, US), Tsu-0 (Tsu, Japan), Yo-0 (Yosemite National Park, US). We obtained the At5g23170 region in four overlapping fragments using primer pairs L62 (5’-GCGTCTCAAATTAAACTCTTCTG-3’)/L63 (5’GTCCGAGAGAGAATTAACTCTTC-3’), GR218300 (5’CCGTGAAAACCAACGAGTTC-3’)/GR220300 (5’- GGTCTAGTGGGTTTTGATTTTC3’), 13-3af (5’-CTAAACCCTAAACCCAAAGTTC-3’)/L68 (5’GCTGCATCTCTAAGTCTTTGAG-3’), and L67 (5’-CGATCTATGGCAGTGACAAG3’)/13-4r (5’-CACACTTGTCTCCATTAATCAG-3’). We gel purified PCR products with QiaQuick columns (Qiagen, Germany) and cloned them into pCR 2.1TOPO vectors (Invitrogen, The Netherlands). We sequenced four clones each on an automated Applied Biosystems 3730xl DNA Analyzer using BigDye terminators version 3.1. We used the DNAstar software package (DNASTAR Inc., Madison, WI, USA) for sequence assembly and alignment. Sequence data are deposited at EMBL (accession nos. AJ864968 to AJ864998). 1. Kroymann, J., Donnerhacke, S., Schnabelrauch, D. & Mitchell-Olds, T. Evolutionary dynamics of an Arabidopsis insect resistance QTL. Proc. Natl. Acad. Sci. USA 100, 1458714592 (2003). 2. Kliebenstein, D. J., Lambrix, V. M., Reichelt, M., Gershenzon, J. & Mitchell-Olds, T. Gene duplication in the diversification of secondary metabolism: tandem 2-oxoglutaratedependent dioxygenases control glucosinolate biosynthesis in Arabidopsis. Plant Cell 12, 681-693 (2001). 3. Kroymann, J., Textor, S., Tokuhisa, J. G., Falk, K. L., Bartram, S., Gershenzon, J. & Mitchell-Olds, T. A gene controlling variation in Arabidopsis thaliana glucosinolate composition is part of the methionine chain elongation pathway. Plant Phys. 127, 1077-1088 (2001).