tpj5116_sm_SupportingInformationlegends
advertisement

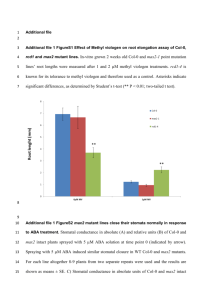
Supporting Information legends Figure S1. (A) A schematic that shows the area of the abaxial leaf surface used for the cryo-SEM and the method adopted for measuring stomatal aperture sizes using Image J software. Three measurements (double arrows): one in the center and the other 2 measurements were taken approximately half way between the end and the center (bar=10µm). (B) Percentage of number of open stomata post Bacillus subtilis FB17 root inoculation. Stomata were counted using images obtained using cryo-SEM randomly at four different regions on the leaf piece (0.5cm2) after 1 and 3 h post root inoculation with B. subtilis FB17. Results shown as mean ± SEM, no. of leaves=10. (C) Stomatal aperture in leaves of Col-0 plants, after foliar dip with COR (0.05-1µM) or water. (D) Stomatal aperture in the leaves of Col-0 plants, leaf dipped with COR (0.05-5µM) and root inoculated with FB17. Results shown as mean±SEM, n=25 stomata. Means of common letters were not significantly different at P ≤ 0.05, Duncan’s Multiple Range Test (DMRT). Figure S2. Confocal micrographs of the abaxial phyllosphere of A. thaliana Col-0 leaves infected by rosette dip with Pseudomonas syringae pv. tomato (PstDC3000) and root inoculated with Bacillus subtilis FB17. The time-lapse series of GFP-tagged P. syringae moving in the phyllosphere was on the Zeiss 5 LIVE line scanning confocal at 10 frames per second and are also shown as real-time movies in Movie S1 and S2. (A) PstDC3000-GFP strain (in green) on the Col-0 leaf surface post 3 h of leaf-dip inoculation. The encircled area in panel A showed open stomata through which PstDC3000 penetrated in a perpendicular entry mode. The last micrographs in panel A showed the apoplastic space (arrow) beneath the open stomata with live PstDC3000 post entry. (B) Leaf surface of Col-0 plants leaf-dipped with PstDC3000 and root inoculated with FB17 post 3 h. The striking difference in between panel A and B reveals increased colonization of PstDC3000 on the leaf surface (in B) presumably as a result of closed stomata (encircled area) (Bar= 20µm). Figure S3. (A) Stomatal apertures in the Col-0 plants after challenge inoculation with PstDC3000 and root inoculation with either FB17 or OP50. FB17 addition significantly reduced the stomatal aperture sizes in the presence of PstDC3000 as opposed to the OP50 inoculation to the roots at 3 h. (B) Reduction in the stomatal aperture sizes after foliar PstDC3000 infection and root treatment with the MAMPs : LPS (lipopolysaccharides) and flg22 (flagellin). The MAMP, flg22 reduced stomatal aperture sizes in the presence of PstDC3000. (C) Dose effect of various LPS concentrations on stomatal aperture after addition to the roots of Col-0 plants (D) Dose effect of various flg22 concentrations on stomatal aperture after addition to the roots of Col-0 plants. (E) FLS2 expression in the roots of Col-0 plants after root inoculation with FB17, Pf0-1, and OP50. Mock consisted of sterile distilled water and 1µM flg22 was added to the roots as the positive control. Means of common letters were not significantly different at P ≤ 0.05, Duncan’s Multiple Range Test (DMRT). Figure S4. (A) Confocal micrographs of the bacterial colonization in the roots of in vitro raised Col-0 plants. B. subtilis FB17 (~0.02 OD600) was monitored with SYTO13® (green) on A. thaliana Col-0 roots (red auto-fluorescence) by confocal microscopy. No biofilm formation was observed in the control roots. Biofilms formed after 24 h post root inoculation with FB17, and more biofilms were formed when FB17 and PstDC3000 were co-inoculated (bar=20 µm). (B) Multiplication of FB17 in the roots of FB17 root inoculated and PstDC3000 inoculated plants represented as colony forming units. Pellet grown plants were root inoculated for 48 h with FB17 and foliar challenged with PstDC3000. Means of common letters were not significantly different at P ≤ 0.05, according to Duncan’s Multiple Range Test. Figure S5. Root inoculation with Bacillus subtilis FB17 affects the stomatal conductance, transpiration rate in the A. thaliana (Col-0) plants. (A, B) Typical time course changes of the stomatal conductance and transpiration rate in the abaxial leaf surface of the Col-0 plants exposed to the diurnal changes. FB17 was inoculated to the roots of the plants. The measurements indicate an overall reduction in the stomatal conductance and the leaf transpiration rate after exposure of roots to FB17. Clock time refers to the time the measurements were taken and between each measurement there was a 4 h time interval constituting an entire 24 h light-dark-light photoperiod. Error bar indicates the standard error of mean (no. of plants=10). Figure S6. Reverse transcription and polymerase chain reaction (RT-PCR) of the RNA isolated from the leaves of Col-0 seedlings after root treatment with B. subtilis FB17 (~0.5 OD600). RT-PCR was performed with the primers (see supplementary methods, data S1) specific for the ABA biosynthetic genes, aba1 and nced2. Note that in the FB17 treated Col-0 seedlings the expression patterns of the above mentioned genes was higher with FB17 as compared to other B. subtilis strains and E.coli OP50. To further validate the extraction procedure under the same light conditions, the extracted RNA was subjected to RT-PCR for the Arabidopsis circadian system genes toc1 (timing of cab expression1) and cca1 (circadian clock associated1). Each band was normalized against the intensity obtained with the same cDNA using the ubq1 primers. Figure S7. (A) Stomatal apertures in mutants of ABA biosynthetic pathway. Mutants aba1 and aba3-1 showed significant reduction in stomatal aperture sizes as opposed to aba2-1. (B) Stomatal apertures in the SA signaling mutant, npr1-1 post FB17 root inoculation. Means of common letters were not significantly different at P ≤ 0.05, Duncan’s Multiple Range Test (DMRT). Figure S8. PR1 gene induction in the Col-0 (A) and ost1-1 (B) plants after FB17 root inoculation or foliar PstDC3000 inoculation or co-inoculation on a time course basis. Means of common letters were not significantly different at P ≤ 0.05, Duncan’s Multiple Range Test (DMRT). Figure S9. (A) Disease symptoms in Col-0 leaf (top panel) and whole plant (bottom panel) after PstDC3000, FB17 + PstDC3000, SA+PstDC3000, ABA+PstDC3000 and SA+ABA+PstDC3000 coinoculation/treatments. Post 72h of inoculation/treatment visual disease symptoms were recorded. (B) Stomatal apertures in the aba2-1 plants after root inoculation/treatment with ABA and or SA and FB17. Biosynthetic mutants aba2-1 is compromised when reciprocal hormones are added to the roots thus causing a decrease in the stomatal apertures (n=44 stomata). (C) PstDC3000 titers in the Col-0 and aba21 plants. Data shown as mean ± SEM, n=12. Means of common letters were not significantly different at P ≤ 0.05, Duncan’s Multiple Range Test (DMRT). Figure S10. (A) PstDC3000 titers in the ABA, SA, ET biosynthetic and signaling pathway mutants and transgenic NahG plants with or without FB17 root inoculation. Data shown as mean ± SEM, n=12; * P ≤ 0.05, ** P ≤ 0.01, ns=non-significant, two tailed‘t’ test. (B) PstDC3000 titers in the ABA biosynthetic mutants with and without FB17 root inoculation. Data shown as mean ± SEM, n=12; *P≤0.05, two tailed‘t’ test. (C) Stomatal apertures in the etr1-1, and the ethylene constitutive mutant, ctr1-1, were measured at 1 and 3 h post root inoculation of FB17. FB17-mediated stomatal closure was abrogated at 1 h in etr1-1 and enhanced in ctr1-1. Stomatal closure was unaffected in the mutants at 3 h. Results shown as mean ± SEM, n=25 stomata. Means of common letters were not significantly different at P ≤ 0.05, Duncan’s Multiple Range Test (DMRT). Movie S1. Live confocal micrograph of the abaxial leaf surface of A. thaliana Col-0 after infection with GFP-tagged PstDC3000. Movie S2. Live confocal micrograph of the abaxial leaf surface of A. thaliana Col-0 after infection with GFP-tagged PstDC3000 and root inoculation with B. subtilis FB17. Methods S1. Supporting experimental procedures.