CH 30 – Nuclear Energy and Elementary Particles
advertisement
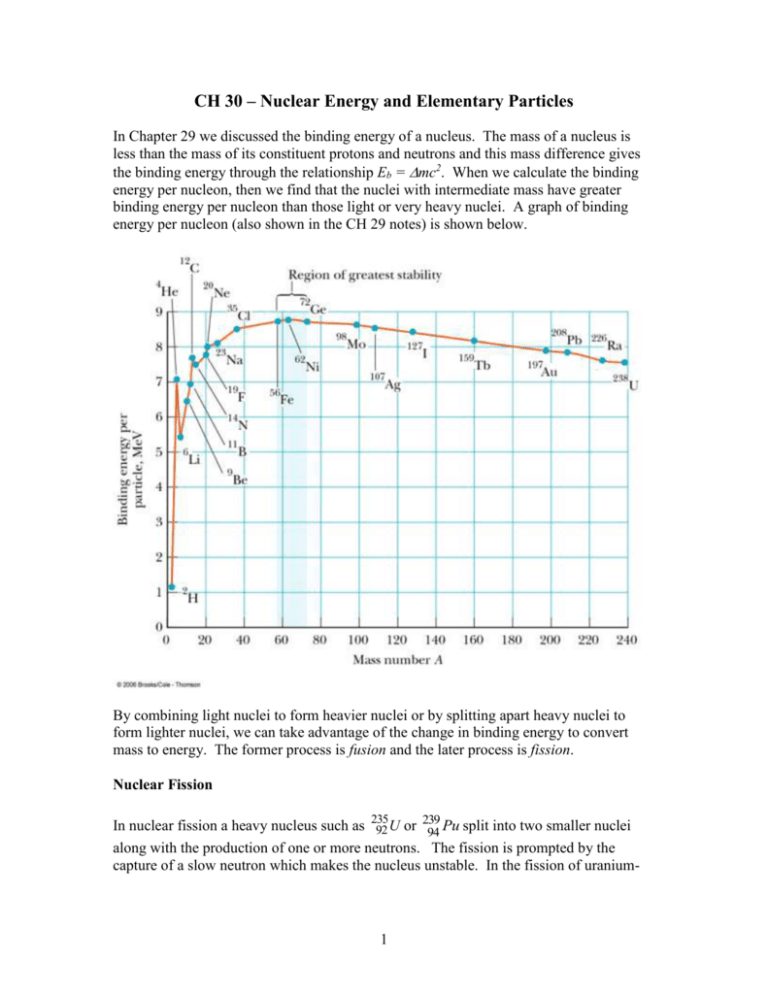
CH 30 – Nuclear Energy and Elementary Particles In Chapter 29 we discussed the binding energy of a nucleus. The mass of a nucleus is less than the mass of its constituent protons and neutrons and this mass difference gives the binding energy through the relationship Eb = mc2. When we calculate the binding energy per nucleon, then we find that the nuclei with intermediate mass have greater binding energy per nucleon than those light or very heavy nuclei. A graph of binding energy per nucleon (also shown in the CH 29 notes) is shown below. By combining light nuclei to form heavier nuclei or by splitting apart heavy nuclei to form lighter nuclei, we can take advantage of the change in binding energy to convert mass to energy. The former process is fusion and the later process is fission. Nuclear Fission 239 In nuclear fission a heavy nucleus such as 235 92 U or 94 Pu split into two smaller nuclei along with the production of one or more neutrons. The fission is prompted by the capture of a slow neutron which makes the nucleus unstable. In the fission of uranium- 1 235, the capture of a neutron forms uranium-236 in an excited state which subsequently breaks apart as shown below. 1 235 236 * 0 n 92 U 92 U X Y neutrons The product nuclei produced in nuclear fission are typically radioactive. There are many possible fission products. Some specific examples for uranium-235 are 1 235 141 92 1 0 n 92 U 56 Ba 36 Kr 30 n 1 235 140 94 1 0 n 92 U 54 Xe 38 Sr 2 0 n An estimate of the energy released in a typical fission event can be determined as follows. From the above graph, we see that the binding energy per nucleon for U-238 is about 7.5 Mev and the binding energy per nucleon for a nucleus with A = 119 (= 238/2) is about 8.5 Mev. So, the energy released per nucleon is about 1 Mev, and the total energy released is about 238 Mev. A more precise value is Q 208 Mev. This lower value is in part because the binding energy per nuclei of the products is smaller than 8.5 Mev since they are unstable and not typical of the values shown on the graph. Example: Calculate the total energy released in the fission of 1 g of U-235. Solution: We calculate the total number of atoms in 1 g of U-235 and multiply this by 208 Mev. N nN A 1g 6.02 x10 23 / mole 2.56 x10 21 235 g / mole E NQ ( 2.56 x10 21 )( 208Mev ) ( 5.33x10 29 ev )( 1.6 x10 19 J / ev ) 8.53x1010 J Example: A typical US household uses 1000 W of electricity. How long would the energy released in the fission of 1 g of U-235 power a household if all the energy could be converted in to electricity? 2 Answer: t E 8.53x1010 J 8.53x107 s P 1000 J / s Converting to years – t ( 8.53x10 7 s )( 1yr ) 2.7 yr 24 x3600 x365s Nuclear Reactors A nuclear reactor is designed to allow the sustained fission through a chain reaction. The fission of a U-235 nucleus produces neutrons which can, in turn, be absorbed by other nearby U-235 nuclei. On average each fission event produces about 2.5 neutrons. If an average of one of these neutrons prompts another fission event, then this will go on until all the U-235 nuclei have been depleted. The chain reaction is said to be critical. If more than one neutron from each fission event prompts another fission event, then the number of nuclei undergoing fission will rapidly increase in time and the chain reaction is super critical. The rate at which energy is released will rapidly increase and can lead to a nuclear explosion of a melt-down of the reactor. On the other hand, if an average of less than one neutron produced in a fission event leads to another fission event, then the chain reaction is not self-sustaining and is subcritical. The figure below illustrates a super critical chain reaction. 3 The neutrons released in a fission event have a kinetic energy of about 2 Mev. This energy is too large to be absorbed by another U-235 nucleus and initiate another fission event. A reactor contains a moderator material that slows down the neutrons. A typical moderator material is “heavy water”, D2O, where D is deuterium. An illustration of a reactor core is shown to the right. Reactors are designed to operate near critical conditions. To prevent a reactor from going super critical control rods are inserted between fuel elements. The control rods are made of a material such as cadmium that is a good absorber of neutrons. The control rods can be moved up and down as needed to keep the reactor operating near the critical condition. The energy released in nuclear reactors is in the form of heat. A heat exchanger is used to generate steam which drives a turbine and produces electricity. Nuclear Fusion In nuclear fusion lighter nuclei combine to form heavier nuclei. For A less than 60 or so the heavy nuclei have a greater binding energy per nuclei than the light nuclei, so fusion results in a decrease in mass with the mass difference appearing as energy released in the process. Nuclear fusion requires overcoming the electrostatic repulsion between the nuclei. This requires that the nuclei just collide at very high speeds, which means very high temperatures. Nuclear Fusion in the Sun Nuclear fusion is the source of the radiation emitted by the sun and the stars. Stars are formed by the gravitational collapse of dust and gas. At the gas collapses inward the molecules gain speed and the temperature rapidly increases. Eventually when the temperature becomes sufficiently large fusion takes place and energy is released. The collapse of the gas (plasma) eventually ceases when the pressure due to the thermal motion of the particles and the radiation pressure become sufficient to balance the pressure due to the gravitational force. For the sun and other stars that are rich in hydrogen, protons fuse to form deuterons with the release of a positron and a neutrino: 1 1 2 1 H 1H 1 D e Fusion takes place in the interior of the sun, where the core temperature is about 107 K. The surface temperature is about 5,800 K, which is too low for fusion. Most collisions 4 between protons in the interior do not result in fusion, but the fusion process is aided by the very high density in the core. Additional fusion reactions in the sun eventually produce helium-4: 1 2 3 1 H 1 D 2 He 1 3 4 1 H 2 He 2 He e 3 3 4 1 2 He 2 He 2 He 2 1H At the earth’s surface, the neutrino flux from the sun is very large, about 1011/cm2/s. They interact very, very weakly with matter, so almost all the neutrinos that strike the earth (and you) pass through undetected. Fusion Reactors Extensive research has been done and is continuing on the design of fusion reactors for converting mass into energy. Fusion has many potential advantages over fission for energy production. The mass change per nucleon in fusion is much greater than in fission and there is little long-lived radioactive waste as a byproduct to worry about. In addition, deuterium, a good fuel for fusion, is relatively abundant in nature and can be cheaply extracted from water. The problem is how to produce the conditions such that significant fusion can take place in a controllable manner in which energy can be usefully extracted. A hydrogen bomb is an uncontrolled fusion reaction. To emulate the density and temperature at the sun’s interior would require extreme pressure. Additionally, no material could contain the high temperature plasma without evaporating. A tokomak is a device that makes use of magnetic fields to confine a high temperature plasma. Since the density of the plasma is much less than the density of the sun’s core, the temperature of the plasma must be much larger than the sun’s core, ~ 108 K. In order for a fusion reactor employing a high-temperature plasma to be viable, the energy released in the fusion process must exceed the energy required to heat the plasma to the necessary temperature and maintain it at this temperature for a sufficient time. The amount of time that the plasma must be held at the reaction temperature depends on the density of the plasma. The international community has agreed to build a new tokomak (ITER) in France that would generate 10 times more energy than required to maintain the temperature of the plasma at fusion conditions (http://www.iter.org/ ). The goal is to demonstrate the viability of a fusion reactor. 5 Example: Calculate the energy released in the following fusion reactions. 2 2 3 1 1 D 1 D 1T 1 H 2 3 4 1 1 D 1T 2 He 0 n Solution: For the first reaction, m 2md mT mH 2( 2.014102 u ) 3.016049 u 1.007825 u 0.00433 u Q mc 2 ( 0.00433u )( 931.5Mev / c 2u )c 2 4.03Mev For the second reaction, m md mT mHe 3 mn 2.014102 u 3.016049 u 4.002603 u 1.008665 u 0.01888 u Q mc 2 ( 0.01888u )( 931.5Mev / c 2u )c 2 17.6Mev The energy released in the second reaction is considerably more than in the first, but it requires tritium, which is very rare. The moon, however, is thought to contain a significant amount of tritium, and mining the moon for this fusion fuel has been proposed. An alternative concept for a controlled fusion reactor is inertial laser confinement fusion. In this approach a small pellet containing deuterium and tritium is blasted simultaneously from different angles by a large number of laser pulses. The hope is that the pulses can generate sufficient pressure on the pellet so that the density and temperature reach fusion conditions. Research on this approach is taking place at Lawrence Livermore National Laboratory (https://lasers.llnl.gov ). Elementary Particles At one time it was thought that the most elementary particle was the atom. Then it was assumed that the elementary particles consisted of the electron, proton, neutron, and photon. It is now known that a large variety of short-lived sub-atomic particles can be generated by high energy collisions of other particles and by the decay of other shortlived particles. Physicists are continually trying to determine which of these particles are the basic building blocks of nature. That is, which are truly ‘elementary’. 6 An important property of particles is the force that they can experience. There are four basic forces in nature, given in the table below. Force Strong Electromagnetic Weak Gravitational Relative Strength 1 10-2 10-6 10-43 Range Short (~ 1 fm) Long (~ 1/r2) Sort (~10-3 fm) Long (~ 1/r2) Mediating Field Particle Gluon Photon W ±, Z Graviton The forces vary widely in strength. The strongest is the short range “strong force” that acts between nucleons in the nucleus. The “electromagnetic force” is the electric and magnetic force that is based on a particle’s charge and is long-range. The “weak” force is a short range force that is involved in beta decay. The “gravitational” force is the weakest of all and is long range. It is believed that these forces are mediated by the exchange of ‘virtual’ particles listed in the right column of the table. This would be somewhat analogous to the bonding of the two protons in a hydrogen molecule that is caused by the ‘exchange’ of the two orbital electrons. When two particles interact by one of these fundamental forces, a virtual field particle is being continually emitted by one particle and absorbed by the other. It is called virtual since it is never directly detected. These virtual particles only exist during the short time during which they are being exchanged. The existence and non-existence of these particles would seem to violate conservation of energy; however, the uncertainty principle, Et /2, allows this to happen to a degree that depends on the time during which the energy is determined. Because of the short range of the interactions and the speed of the virtual particles, their lifetime can be short and the energies of the virtual particles can be significant. Classifications of Particles All particles other than the photon can be classified as either a lepton or a hadron. Leptons The leptons include the electron (e-) , muon (-) , and tau ( -) particles and their associated neutrinos - e, , . These six particles also have their antiparticles. All but the muon and tau particle are stable. The muon and tau masses are about 207 and 3490 times the electron mass. The leptons have a spin of ½. 7 Hadrons The hadrons include mesons and baryons. Mesons There are a large number of mesons with a wide range of masses. Some have charge and some don’t. Examples of mesons are the pion (0, -, +) and the kaon (K0, K-, K+). Mesons have integer spin and all are unstable. Baryons There are also a large number of baryons. The proton and the neutron are baryons. Others include the lambda (0) and sigma (0, -, +) particles. Baryons have halfinteger spin (1/2, 3/2, …), and all are unstable except the proton. A free neutron has a lifetime of about 15 min. When bound in the nucleus it is stable. Particles can also be classified by their spin. Half-integer spin particles (leptons and baryons) are referred to as fermions. Integer spin particles (0, 1, 2, ..) are referred to as bosons. The mesons and the photon (spin = 1) are bosons. Quarks The leptons are considered to be elementary particles. The have no internal structure. The hadrons, however, are not considered to be elementary particles and are thought to consist of bound states of quarks. A quark has a charge of 1/3e or 2/3e. There are six types of quarks and their antiparticles: up (u), down (d), charmed (c), strange (s), top (t), and bottom (b). Quarks are fermions with a spin of ½. Isolated quarks have never been observed. The mass and charge of the quarks are listed below. The antiquarks have opposite signs. Quark u d c s t b Rest mass energy 360 360 1500 540 173 5 Charge (e) +2/3 -1/3 +2/3 -1/3 +2/3 -1/3 Mesons consist of 2 quarks and baryons consist of 3 quarks. The composition of the proton is uud . Its charge is 2/3e + 2/3e – 1/3e = e. The neutron is udd and its charge is 2/3e - 1/3e – 1/3e = 0. The + is du and its charge is 1/3e+2/3e = e. 8