BALMER LINES OF HYDROGEN
advertisement

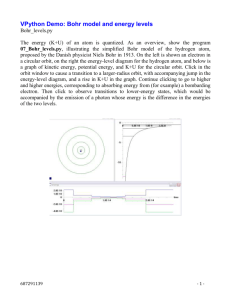
Physics 290 Quantum Physics Fall 2004 BALMER LINES OF HYDROGEN INTRODUCTION As far back as the early 19th century it was known that, when a pure material is heated so much that it becomes a glowing gas, the emitted light is composed of certain very definite wavelengths (colors). Since the advent of quantum mechanics we can understand these patterns of wavelengths as light emitted when electrons make transitions from one allowed atomic energy state to another. By measuring the emitted wavelengths of light, we can learn about the allowed energies of the electrons in atoms. In this experiment you will send light from a hydrogen gas lamp through a diffraction grating in order to determine which visible wavelengths which are present. The diffraction grating is a series of closely spaced lines, etched in glass, which act like multiple slits. Remember that monochromatic light passing through the slits forms a pattern (spots of constructive interference) on a nearby screen, according to the expression: dsin = m. m = ±1,±2,±3…… (1) If many wavelengths were to be incident on the slits at the same time, then, according to eq. (1), the individual wavelengths will show constructive interference at different 's. A spectroscope (spectrometer is another name) can be used to measure the angles where different colors are seen, and since we know the spacing between lines of the grating, we can determine the wavelengths of the various colors in the spectrum. (Our gratings have 604 lines/mm.) Hydrogen is the simplest atom, consisting of one proton and one electron, and it has the simplest spectrum of all atoms and molecules. In 1885, J. J. Balmer found a remarkable empirical formula (empirical: based only upon the experimental evidence) that completely characterizes the wavelengths of the four visible lines emitted by hydrogen: red, blue-green and two lines in the violet. The formula is 1 1 1 = R 2 2 2 n (2) where n=3, 4,5 and 6 produce the visible wavelengths that we see. R is an experimentally obtained constant called "Rydberg's constant." At the time, there was no theory to justify this formula. Incidentally there are also ultraviolet lines called the Lyman series, but these are beyond the ability of the eye to detect. Other detectors, like photographic plates that were available in Balmer's time, were used to measure the wavelengths of these spectral lines. Early in the 20th century, the Bohr model explained the experimental hydrogen spectrum. In hot hydrogen gas, the atoms are usually in the n=1 ground state but are moving rapidly. When two atoms collide with enough energy, one of the electrons may be knocked to a higher orbit. In your lamp, electrons are accelerated and knock the atoms into excited states. A short while later, the electron will suddenly and spontaneously jump to a lower energy orbit, emitting a photon. Physics 290 Quantum Physics Fall 2004 Because energy is conserved in this "quantum jump," and the electron loses energy in jumping from a higher to a lower orbit, the photon carries away the difference in energy E between these two energy levels. Planck told us how to relate a unique photon energy E to a unique frequency (color, wavelength). Bohr showed that the visible photons emitted by hydrogen are due to electrons jumping to the n=2 orbit from the n=3 - 6 orbits. In fact, his theory requires that any light emitted from hydrogen must be one of the following energies: E k 2e4 1 1 2 1 2 1 2 1 m c m c e e n2 n2 2 n2 n2 2 2 c 2 i i f f (ni >nf are positive integers) (3) due to a jump from the initial (i) to the final (f) orbit. Thus Bohr completely explained Balmer's formula (2), including obtaining an expression for R in terms of fundamental constants of nature. PROCEDURE: ANALYSIS OF THE HYDROGEN SPECTRUM You will be able to see the first order (m=1) and (hopefully) second order (m=2) lines on both sides of the image of the slit. Because of slight errors in alignment of the spectroscope, it is most accurate to determine the angle at which a line occurs by measuring the angle of the line at 1 the left and the corresponding angle at the right; then use sin = 2 (sinL + sinR) . Don't lose accuracy by being careless either in your experimental technique or in your calculations. Be careful with SIG FIGS. The preliminary adjustments of the spectrometer, which are rather technical and exacting, have been made for you. The spectrometer has been adjusted and focused, and the grating has been set at right angles to the incident light. Your job is to set the cross hairs of the telescope on each spectral line in turn. Each position of the telescope will then be read on the circular scale with the aid of the vernier scale, and the angles can be computed from the difference of angles. Observe the spectra from hydrogen gas, and make measurements of the angles in first and second orders. Calculate the wavelengths. Use these data to graphically verify the functional form of Balmer's formula. This experiment is capable of giving very accurate results. Think carefully about how to analyze the data and estimate the errors associated with your results. Compare your measured value for the Rydberg constant R (with error.) with the one obtained from Bohr’s model. What small effect have we neglected in calculating the energies for the Bohr atom? For reference, me = 510.999 keV/c2 and ħc = 197.327 eV nm, and = 1/137.0360, and = m/(1+m/M).