Bohr_model_and_energy_levels
advertisement

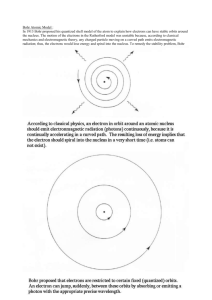
VPython Demo: Bohr model and energy levels Bohr_levels.py The energy (K+U) of an atom is quantized. As an overview, show the program 07_Bohr_levels.py, illustrating the simplified Bohr model of the hydrogen atom, proposed by the Danish physicist Niels Bohr in 1913. On the left is shown an electron in a circular orbit, on the right the energy-level diagram for the hydrogen atom, and below is a graph of kinetic energy, potential energy, and K+U for the circular orbit. Click in the orbit window to cause a transition to a larger-radius orbit, with accompanying jump in the energy-level diagram, and a rise in K+U in the graph. Continue clicking to go to higher and higher energies, corresponding to absorbing energy from (for example) a bombarding electron. Then click to observe transitions to lower-energy states, which would be accompanied by the emission of a photon whose energy is the difference in the energies of the two levels. 687291139 -1- -0.85 eV -1.5 eV -0.28 eV -0.54 eV-0.38 eV -3.4 eV Horizontal is meaningless -13.6 eV From solving Schrodinger Equation (one of two simple systems): EN K U e 13.6 eV , N 1, 2,... N2 After the full development of quantum mechanics, it became clear that the Bohr model for the hydrogen atom is too simple to capture many important features of hydrogen. For example, instead of Bohr's circular orbits, quantum mechanics predicts that the electron exists as a probability cloud around the proton. Yet quantum mechanics predicts that in higher-energy states the electron is on average farther from the proton, just as predicted by the Bohr model. And Bohr's proposed “jumps” between energy levels, with photon emission, does capture the main points about energy quantization. An important drawing convention: Often we only care about the discrete energy levels and just draw horizontal lines without drawing the potential energy curve U. In this case the position along the x-axis is essentially meaningless. For example, we can draw the energy-level scheme for atomic hydrogen as a set of horizontal lines of equal length, disregarding the additional information about maximum separation shown when we display the U curve. 687291139 -2-