Physics 211 Lab 1 - Personal.psu.edu
advertisement

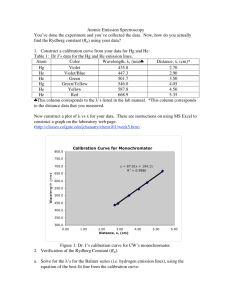
Physics Lab The Hydrogen Spectrum and the Bohr Model 1 Introduction In this lab, we use our grating spectrometer to make precise measurements of the wavelengths of the spectral lines of Hydrogen. Using these, plus Balmer’s formula and the Bohr model, we can find the Rydberg constant and the energy levels of the Hydrogen atom. Procedure Part I: Setting up the spectrometer Follow the instructions for Parts I and II of the previous lab, “Grating Spectrometer,” with the exception that we will use a Hydrogen spectrum tube, rather than Helium. Part II: Collecting Data Follow the instructions for Part III of the previous lab, with the exception that you should try to observe all lines that are in the visible range of the spectrum, for m=1, m=2 and m=3. You should be able to see clearly at least four lines; you may be able to see many more (with most of those in the violet range of the spectrum). If you can not see any of the lines at any of the orders, be sure to note that clearly in the data section of your report. Analysis 1. Calculate the average of your left and right angles for each line (a column for this is included in the data table). 2. Use the average angle, the order, and the grating spacing in the grating equation, to calculate the wavelength in each case. (Note that you only have to show one such calculation, just show results for the rest.) 3. Average the wavelengths that you found for that line in the first order, the same line in the second order, and the same line in the third order (where visible). 4. Use Balmer’s formula, and your data, to calculate the Rydberg constant. Do this for each of your spectral lines (the Hα, Hβ, Hγ, etc.). Then, find the average of your Rydberg constant results. 5. Calculate the Rydberg constant from fundamental quantities, as discussed in the text. 6. Calculate the % error for your average from step 4, vs. the accepted value found in step 5. Results Give a table listing your calculated wavelengths from Analysis step 2 above, the average wavelength from step 3, the Rydberg constants from step 4, the average Rydberg constant from your data from step 4, the theoretical Rydberg constant from step 5, and the % error from step 6. Questions 1. Are the theoretical Balmer wavelengths consistent with your measured wavelengths? Why or why not? 2. Are you surprised by the accuracy of your results? Why or why not? Physics Lab 3. The Hydrogen Spectrum and the Bohr Model 2 Sketch an energy level diagram of the hydrogen atom with the various levels labeled with the proper value of the quantum number n. Indicate on your diagram which transitions cause the lines you observed in the Balmer series. Reference Information: (From: http://www.solarobserving.com/halpha.htm) Color Wavelength Relative (nm) Intensity Transition Name Red 656.28 180 n=3 → n=2 Hα Cyan 486.13 80 n=4 → n=2 Hβ Blue-Violet 434.05 30 n=5 → n=2 Hγ Violet 410.17 15 n=6 → n=2 Hδ Violet 397.01 8 n=7 → n=2 Hε Violet 388.90 6 n=8 → n=2 Hζ Violet 383.54 5 n=9 → n=2 Hη (Adapted from: http://hyperphysics.phy-astr.gsu.edu/hbase/hyde.html) Physics Lab The Hydrogen Spectrum and the Bohr Model 3 Data Table Reference Angle (if necessary):___________ Color, Side , Right , Left , Right , Left , Right , Left , Right , Left , Right , Left , Right , Left , Right , Left , Right , Left , Right , Left , Right , Left , Right , Left , Right , Left , Right , Left , Right , Left Brightness Order (m) Measured Angle Difference Angle (if necessary) Average Angle