Objectives for Nuclear Chemistry
advertisement

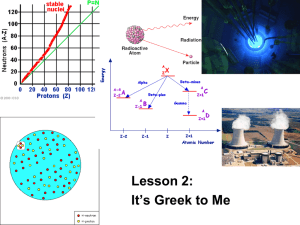
Objectives for Nuclear Chemistry 1) 2) 3) 4) Define radioactivity and distinguish between natural and artificial Explain transmutation List 3 radioactive emissions: alpha, beta, gamma Describe what each emission is composed of and how they differ from each other with respect to mass, charge, penetrating power, and ionizing power 5) Tell what happens to an element that undergoes alpha decay, beta decay, or gamma decay 6) Discuss the process used to separate the three types of radioactive emissions. 7) Define half life and use Table N to find the half lives of various radioactive isotopes 8) Calculate half life and fraction remaining from various word problems 9) Solve for unknowns in nuclear equations 10) Define and explain mass defect 11) Define binding energy 12) Explain the basic difference between a fission reaction and a fusion reaction 13) Explain how a chain reaction works 14) Discuss the difference between a fission reaction in a nuclear bomb and the one in a nuclear fission reactor 15) Give the details of a fusion reaction 16) List the three places fusion occurs: fusion reactors, the sun, hydrogen bomb Objective 1 Radioactivity: Isotopes of elements may be stable or not, it depends on the number of protons and neutrons in the nucleus of the isotope. The unstable nuclei are said to be radioactive and decay emitting radiation There is natural and artificial radioactivity. Natural radioactivity is when a nucleus spontaneously decays into its product. In artificial radioactivity, the nucleus must be hit with a particle in order to make it unstable and cause it to emit radiation. The way to identify artificial vs. natural when given a reaction is that an equation which represents natural radioactive decay, there is only one reactant. In artificial experiments, there must be more than one reactant. There is something known as the “belt of stability.” This belt relates the ratio of protons and neutrons to the stability of the atom. Atoms which have either too high or too small of a ratio are unstable and therefore radioactively decay to obtain a ratio which is within the belt. Radioactivity: when nuclei decay, emitting radiation Stability depends on the ratio of protons/neutrons Natural vs artificial radioactivity o Natural one reactant - All on its own o Artificial two reactants – man made The Belt of Stability Objective 2 When a nucleus is changed and the atom is converted from one atom to another, this is called transmutation. This can be accomplished either naturally or artificially by the bombardment of the element with high-energy particles. Some isotopes are naturally unstable. Radium-226, Thalium-234 are both unstable and they decay by different methods. Some isotopes are stable until they are bombarded by neutrons in a nuclear reactor, the new isotope is radioactive and therefore decays. Transmutation: one atom becoming an atom of a different element Some transmutate spontaneously, others only artificially Objective 3 The three types of emissions are alpha, beta, and gamma. They are listed with some of their properties on Table O in the Reference Tables Knowledge of the three types of particles is essential – know how to use the reference tables Table O shows all emissions Objective 4 Alpha particles: composed of a helium nucleus, they have an atomic mass of 4 and an atomic number of 2. They have a positive charge. They are the weakest in terms of penetrating or ionizing power. Beta particles: explained as the emission of an electron, beta particles are the intermediate radiation in terms of power. Beta particles have a negative charge and no mass. Gamma: pure energy. Gamma radiation has no charge and no mass. Particle Name Symbol Alpha 4 2 0 1 0 0 Beta Gamma or 0 1 e Charge Mass 4 Atomic Number 2 Penetrating Power Low + - 0 -1 Medium 0 0 0 High Objective 5 Alpha particles: The release of an alpha particle causes a nucleus to decrease its atomic number by 2 and its atomic mass by 4. This is a transmutation in which the atom becomes a new element, two spaces earlier on the periodic table. Beta Particle: The emission of a beta particle causes an element to increase its atomic number by 1. One way to think of this is that a neutron splits off an electron, creating a positive particle – a proton. This causes the atomic number to increase by one, there is a new proton, and the atomic mass remains the same, the total number of protons and neutrons remains the same. Gamma: Causes no change in the atomic structure of the atom by its departure. It is usually given off as one of the products in a nuclear reaction. Gamma radiation is pure energy. Alpha o Mass = 4; A# = 2 o Element becomes 2 less A# Beta o Element increases A# by 1 o Mass stays same Gamma o Pure energy – no mass Objective 6 A mixture of the three types of radiation may be separated by passing them through a magnetic field. The alpha particles have a positive charge and will therefore be drawn toward the negative side of the field. The beta particles are negative and will be pulled to the positive side of the magnet. The gamma radiation has no charge and will therefore pass straight through the magnetic without being affected. Alpha deflects to negative side Gamma goes straight Beta deflects to positive side Objective 7 The half life of an isotope is the amount of time it takes for half of a sample to decay. Half lives are specific to the isotope being observed and half lives are therefore useful in the analysis of an unknown sample and can be used to determine the identity of the unknown. Potassium-37 has a half life of 1.23 s and decays by + (positron) Strontium-90 has a half life of 28.1 y and decays by - (beta) Radon-226 has a half life of 1600 y and decays by (alpha) A radioactive sample decays so that only half of its original mass remains, this takes 8.07 days. What is the identity of the isotope, and what is its method of decay? (131I; beta) ½ life = time for half to disappear Table N Different modes of decay listed too Objective 8 If you know the amount of time it takes for a certain amount of an isotope to decay then you can calculate the half life of the isotope. By using the fraction remaining provided in a problem you can determine how many half lives have elapsed. Once you know the number of half lives, divide the total time by the number of half lives to determine the time for one half life. Alternatively, you may be given the half life information and be asked how long it will take to get to a certain fraction remaining. Using the fraction remaining that you are given, determine how many half lives need to elapse to get to that point. Multiply the number of half lives by the time for one half life to get the total time necessary. 1. How many half lives must a sample go through before there is only 1/8 of its original mass remaining? (3) 2. How much time has passed if iodine-131 has gone through 3 half lives? (24 d) 3. What fraction remains after two half lives? (1/4) 4. How long will it take for 100 grams of neon-19 to decay to 3.125 g? (86 s) 5. A sample takes 112.4 y for a sample of radioactive metal to decay to 1/16th its original mass. What isotope is being observed? (strontium-90) Objective 9 In nuclear equations the total of the atomic masses of the products must equal the total atomic masses of the reactants. The same must be true for the atomic numbers of the substances on the product and the reactant side of the equation. Write nuclear equations for the decay of the following isotopes according to the decay modes listed in your Reference Tables: N-16: 16N 16O + -1e Pu-239: 239 94Pu 235 92U + 4 2He Fe-53: 5326Fe 5325Mn + +1 Objective 10 Mass defect is the difference between the predicted value of the mass of an atom and its actual observed mass. The calculated mass is derived from adding the masses of protons and neutrons that are present. The actual mass is less than this. The difference is due to the fact that the formation of a nucleus releases a tremendous amount of energy. Objective 11 The energy released in the formation of an atom is known as the binding energy. Einstein’s equation for energy, E = mc2 accounts for the change in mass as a result of the release of the binding energy. Objective 12 Fission reactions result in the splitting of heavier nuclei into lighter ones. This is accomplished by hitting the large nucleus with a slow moving neutron, the nucleus absorbs the neutron and becomes unstable. The new unstable nucleus then breaks apart into fission fragments of elements of lighter weight, releases energy, and two or more neutrons. The release of energy comes from the conversion of matter to energy according to Einstein’s equation. Objective 13 A chain reaction is a self-sustaining reaction. This means that its products cause the reaction to repeat itself using fresh reactants. In the case of nuclear reactions, neutrons which are produced in the fission of a large nucleus then cause the fission of another nucleus. This second nucleus then produces neutrons as its products, these neutrons then go on to make another fission reaction, and so on. Objective 14 The difference between a fission bomb and a fission reactor (nuclear reactor) is that the bomb is uncontrolled. The reactor has components which either remove or slow down the number of available neutrons in the reaction chamber, since the neutrons are necessary to the reaction this controls the rate of the chain reaction. Objective 15 A fusion reaction involves the combination of two small atoms to make one larger atom. The fusion reaction which involves the combination of two hydrogen atoms forms a helium atom. Objective 16 The sun is one big fusion reactor. The hydrogen bomb is a fusion reaction which is allowed to run uncontrolled after having been started by a fission reaction. There are some nuclear reactors which harness the energy of the fusion reaction.