Absolute Dating
advertisement

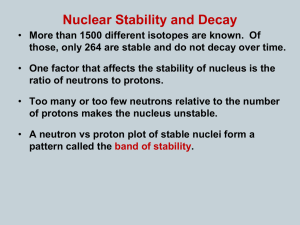
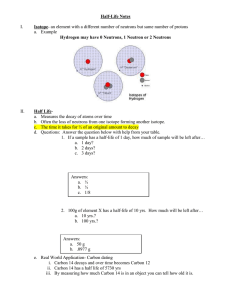
Absolute Dating Chapter 3, Sec.3 Process to find the approximate age of rocks or fossils Atoms Smallest part of matter Made of protons, neutrons, electrons Center called the nucleus Everything is made of atoms Protons, neutrons in nucleus Electrons move around nucleus Every element has a diff. # of protons Isotopes Atoms of the same element with different # of neutrons Radioactive Decay Most atoms are stable Some are unstable and breakdown or decay (radioactive) High energy particles are given off Can damage cells, organs, etc.. X-rays, gamma rays, electrons, protons, neutrons Decay occurs at a constant rate called half-life Half-life The time it takes for one-half of a sample to decay Problem I start with 10 grams of a sample It’s half-life is 20 years After 20 years, how much left? 5 grams After 40 years, how much left 2.5 grams Some unstable isotopes decay into stable isotopes 3 radioactive dating methods Uranium-Lead Method Potassium-Argon Method Carbon-14 Method