Atomic_Structure
advertisement
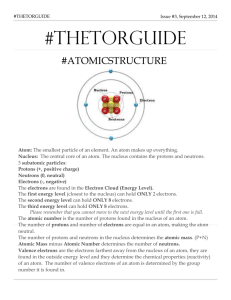
Atomic Structure ATOM • Smallest unit of matter • An element is made up of the same atom ▫ ex: Gold (element on P.T) is made up of gold atoms Parts of an Atom • Atoms are made up of: ▫ Protons (p+): Positive charged ▫ Electrons (e-) : negatively charged ▫ Neutrons (no): no charge (neutral) • These are called subatomic particles • Nucleus is found in the centre of the atom ▫ The two subatomic particles found in the PROTONS nucleus are _________________ and NEUTRONS ______________ • Shells (orbitals) are rings around the nucleus ELECTRONS ▫ ___________________ are located in the shells outside the nucleus. VALENCE SHELL • VALENCE SHELL: outer most shell ▫ Electrons found in the valence shell are called valence electrons Determining number of protons, electrons and neutrons... # protons = ATOMIC NUMBER # electrons = # protons (in a neutral atom) # neutrons = ATOMIC MASS – ATOMIC NUMBER EXAMPLE: Oxygen atomic number = 6 Atomic mass= 16.00 # p= 6 # e= 6 # n= 16 – 6 = 10 Drawing Bohr-Rutherford Diagrams 1) Draw a circle representing the nucleus 2) Draw orbitals (dotted circles) around the nucleus ▫ Hint: the period tells you how many orbitals to draw 3) In the nucleus, indicate how many protons (p+) and neutrons (n0) 4) Fill in the electrons on the orbitals ▫ ▫ 1st orbital: 2e2nd, 3rd, 4th, 5th, orbital – 8 e- Let’s do an example!