Buffer - BowNET
advertisement

Acid-Base Equilibria
Chapter 17
Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 17: Acid-Base Equilibria
I.
II.
Buffer Composition
pH of Buffer Solutions
•
pH after small amounts of acid/base added
III. Designing Buffer Solutions
IV. Acid-Base Titrations
•
Indicator
A buffer solution has the ability to resist large changes in pH
upon the addition of small amounts of either acid or base.
A buffer solution is a solution of:
1. A weak acid and its conjugate base
or
2. A weak base and it conjugate acid
Note: If either the acid or base is a negative
or positive ion; it will be found in the
form of a salt.
Note: It is important that the acid and base
have the conjugate relationship.
16.3
Six Strong Acids
HCl
H2SO4
HBr
HClO4
HI
HNO3
Which of the following are buffer systems? (a) KF/HF
(b) KBr/HBr, (c) Na2CO3/NaHCO3
(a) F- is a weak base and HF is its weak conjugate acid
buffer solution
(b) HBr is a strong acid
not a buffer solution
(c) CO32- is a weak base and HCO3- is it conjugate acid
buffer solution
16.3
How a Buffer Withstands Large Changes in pH
(CH3COO)H/Na(CH3COO-)
How a Buffer Withstands Large Changes in pH
(CH3COO)H/Na(CH3COO-)
Consider an equal molar mixture of CH3COOH and CH3COONa
Add strong acid
H+ (aq) + CH3COO- (aq)
Add strong base
OH- (aq) + CH3COOH (aq)
CH3COOH (aq)
CH3COO- (aq) + H2O (l)
Base of buffer removes excess H+
Acid of buffer removes excess OH-
How a Buffer Withstands Large Changes in pH
(CH3COO)H/Na(CH3COO-)
Introductory Chemistry 2/e by N Tro, Prentice Hall, 2006, pg 495
A buffer solution is the most effective if ...
1) there are large amounts of acid/conjugate base
2) the amounts of acid and conjugate base are
about equal
To find pH of buffer made of; HF/FHF (aq) <-> H+(aq) + F- (aq)
Ka = [H+][F-]
[HF]
H+ = Ka [HF]
[F-]
If the ratio ([HA]/[A-]) stays about same;
pH will not change dramatically !
A buffer solution is the most effective if ...
1) there are large amounts of acid/conjugate base
2) the amounts of acid and conjugate base are about equal
HF
F-
Add
OH-
HF F-
OH- (aq) + HF (aq) <->
F- (aq) + H2O (l)
Free (OH)- reacts with/removes
acid (HF) of buffer and produces
more F-.
Add
H+
HF
F-
H+ (aq) + F-(aq) <-> HF (aq)
Free H+ reacts with/removes
base (F-) of buffer and produces
more HF.
A buffer solution is the most effective if ...
1) there are large amounts of acid/conjugate base
2) the amounts of acid and conjugate base are about equal
FHF
Add
4998 = 0.999
5002
3 = 0.4286
7
OH-
HF F-
5000 = 1
5000
5=1
5
Add
H+
HF
F-
5002 = 1.0008
4998
7 = 2.333
3
Determining the pH
of Buffer Solutions
• The calculations are very similar to
determining the pH of weak acid solutions–
the only difference is that initially there are
both some reactants and products present.
An easier way to determine
pH of Buffer solutions
Henderson-Hasselbalch equation
Consider mixture of salt NaA and weak acid HA.
HA (aq)
H+ (aq) + A- (aq)
[H+][A-]
Ka =
[HA]
[H+]
Ka [HA]
=
[A-]
-log [H+] = -log Ka [HA]
[A-]
[HA]
-log [H+] = -log Ka - log
[A-]
[A-]
pH = pKa + log
[HA]
Henderson-Hasselbalch
equation
[conjugate base]
pH = pKa + log
[weak acid]
[A-]
pH = pKa + log
[HA]
Insignificant Change
Approximation is Assumed
In This Equation!
What is the pH of a solution containing 0.30 M HCOOH
and 0.52 M HCOOK?
Mixture of weak acid and conjugate base!
HCOOH (aq)
Initial (M)
Change (M)
Equilibrium (M)
Common ion effect
0.30 – x 0.30
0.52 + x 0.52
H+ (aq) + HCOO- (aq)
0.30
0.00
0.52
-x
+x
+x
0.30 - x
x
0.52 + x
[HCOO-]
pH = pKa + log
[HCOOH]
[0.52]
= 4.01
pH = 3.77 + log
[0.30]
HCOOH pKa = 3.77
16.2
Calculate the pH of the 0.30 M NH3/0.36 M NH4Cl buffer
system. What is the pH after the addition of 20.0 mL of
0.050 M NaOH to 80.0 mL of the buffer solution?
NH4+ (aq)
[NH3]
pH = pKa + log
[NH4+]
start (moles)
end (moles)
H+ (aq) + NH3 (aq)
pKa = 9.25
0.029
0.001
NH4+ (aq) + OH- (aq)
0.028
0.0
[0.30]
pH = 9.25 + log
= 9.17
[0.36]
0.024
H2O (l) + NH3 (aq)
0.025
final volume = 80.0 mL + 20.0 mL = 100 mL
[NH4
+]
0.028
0.025
=
[NH3] =
0.10
0.10
[0.25]
pH = 9.25 + log
= 9.20
[0.28]
16.3
Designing Buffer Solutions
pH = pKa + log {[X-]/[HX]}
• Buffers are most effective if there
are equal and large amounts of
weak acid and conjugate base.
[X-] = [HX]
• pH = pKa + log (1)
• pH = pKa
pKa of Weak Acids
Weak Acid
pKa
Hydrofluoric Acid
3.15
Benzoic Acid
4.19
Carbonic Acid
6.38
Hydrocyanic Acid
9.31
Titrations
In a titration a solution of accurately known concentration is
added gradually added to another solution of unknown
concentration until the chemical reaction between the two
solutions is complete.
Equivalence point – the point at which the reaction is complete
Indicator – substance that changes color at (or near) the
equivalence point
Slowly add base
to unknown acid
UNTIL
The indicator
changes color
(pink)
4.7
Acid-Base Titrations
Introductory Chemistry 2/e by N Tro,
Prentice Hall, 2006, pg 480
At endpoint/ equivalence point
+
moles H = moles (OH)
MV(acid) = MV(base)
• Two ways to get endpoint/equivalence point
1. Color change
2. Mid-point of steep rise of acid/base titration
curve
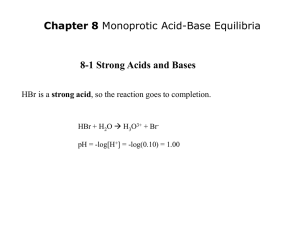
Strong Acid-Strong Base Titrations
NaOH (aq) + HCl (aq)
H2O (l) + NaCl (aq)
neutral salt
0.10 M NaOH added to 25 mL of 0.10 M HCl
16.4
Weak Acid-Strong Base Titrations
CH3COOH (aq) + NaOH (aq)
CH3COONa (aq) + H2O (l)
Basic Salt
At equivalence point (pH > 7):
16.4
Strong Acid-Weak Base Titrations
HCl (aq) + NH3 (aq)
NH4Cl (aq)
Acidic Salt
At equivalence point (pH < 7):
16.4
The titration curve of a strong acid with a strong base.
16.5
Which indicator(s) would you use for a titration of HNO2
with KOH ?
Weak acid titrated with strong base.
At equivalence point, will have conjugate base of weak acid.
At equivalence point, pH > 7
Use cresol red or phenolphthalein
16.5






![[H 2 PO 4 - ] = 0.800 M.](http://s2.studylib.net/store/data/005623813_1-92875a3e2acb84ddbb79ead23a1c6630-300x300.png)