Lec 2 - UMDNJ
advertisement

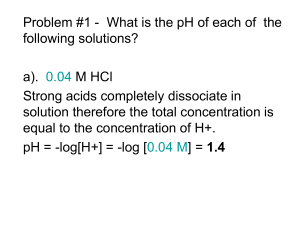
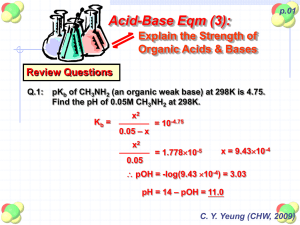
Lecture 2 Dr. Kumar Aid-base balance-Salivary Buffering 1 Buffer solutions Before you start it would be helpful to… know that weak acids and bases are only partly ionised in solution • • be able to calculate pH from hydrogen ion concentration • be able to construct an equation for the dissociation constant of a weak acid Buffer solutions - uses Definition “Solutions which resist changes in pH when small quantities of acid or alkali are added.” Biological Uses In biological systems (saliva, stomach, and blood) it is essential that the pH stays ‘constant’ in order for any metabolic processes to work properly. e.g. If the pH of blood varies by 0.5 it can lead to unconsciousness and coma Most enzymes work best at particular pH values. Other Uses Many household and cosmetic products need to control their pH values. Shampoo Buffer solutions counteract the alkalinity of the soap and prevent irritation Baby lotion Buffer solutions maintain a pH of about 6 to prevent bacteria multiplying Others Washing powder, eye drops, fizzy lemonade Buffer solutions Definition “Solutions which resist changes in pH when small quantities of acid or alkali are added.” Acidic Buffer (pH < 7) potassium salt made from Alkaline Buffer (pH > 7) made from a like ammonium chloride a weak acid + its sodium or weak base Uses Buffering biological systems (e.g. in blood) + its salt Acid-Base Concepts and Buffer Action • • Acids Strong: Tendency to give up a proton readily---HCl HCl + H2O Cl-- + H3O+ Weak: Weak tendency to give up a proton---Acetic Acid CH3COOH + H2O CH3COO-- + H3O+ Bases – Strong—great tendency to accept a proton--- OH ion – Weak--- Poor tendency to accept a proton--CH3-COO- anion Dissociation of a Weak Acid HA H+ + A— (Weak Acid) (proton) (conjugate Base) Ka (acid dissociation constant) = [H+] [A--] [HA] log Ka = log [H+] + log [A--]/[HA] --log [H+] = --log Ka + log [A--]/[HA] pH = pKa + log [conjugate base]/[acid] Henderson- Hasselbalch equation pH = -log[H+] and pKa = --log Ka When [A-]= [HA] pH = pKa + log [HA]/[HA] or pH = pKa + log 1 So pH = pKa Significance of pH and pKa pH = -log [H+] If [H+] = 1 molar (1M) then pH = - log [1] = 0 If [H+] = 10-7 M then pH = -log [10-7]= -(-7)log10 = +7 When [H+] concentration is high pH is a low number When [H+] concentration is low pH is a higher number pH scale is logarithmic and is usually given from 0-14 Calculating the pH of an acidic buffer solution Calculate the pH of a buffer whose [HA] is 0.1 mol/l and [A¯] of 0.1 mol/l. Ka = [H+(aq)] [A¯(aq)] [HA(aq)] re-arrange [H+(aq)] = [HA(aq)] x Ka [A¯(aq)] from information given [A¯] = 0.1 mol/l [HA] = 0.1 mol/l If the Ka of the weak acid HA is 2 x 10-4 mol/l [H+(aq)] = 0.1 x 2 x 10-4 0.1 pH = - log10 [H+(aq)] = 2 x 10-4 mol/l = 3.699 Calculating the pH of an acidic buffer solution Calculate the pH of the solution formed when 500 mlof 0.1 mol /lof weak acid HX is mixed with 500 ml of a 0.2 mol/l solution of its salt NaX. Ka = 4 x 10-5 mol/l Ka = [H+(aq)] [X¯(aq)] [HX(aq)] re-arrange [H+(aq)] = [HX(aq)] Ka [X¯(aq)] The solutions have been mixed; the volume is now 1 litre therefore Substituting [HX] = 0.05 mol /l [X¯] = 0.10 mol/l [H+(aq)] = 0.05 x 4 x 10-5 0.1 pH = - log10 [H+(aq)] and = = 2 x 10-5 mol/l 4.699 Dissociation of water Water is a weak acid and a weak base H2O H+ + OHKw = [H+][OH-] H2 O Kw(H2O) = 10-14 = [H+][OH-] and pH + pOH=14 Since [H+] = [OH-] [H+]2 = 10-14 and [H+] = 10-7 So for water, pH = 7 and pOH = 7 Titration of a weak acid Buffering Capacity of Acid-Conjugate base A buffer is a mixture of weak acid and its conjugate base and its pH should not change appreciably when small amount of acid or base is added CH3-CHOH-COOH CH3-CHOH-COO- + H+ (Lactic acid; pKa=4.5) At pH=4.5 which is equal to pKa, [lactate]=[lactic acid] What will be the change in pH if we add 0.01 M base (OH) to a buffer which contains 0.1 M each of acid and conjugate base? pH = 4.5 + log 0.11/0.09 = 4.5 + log 1.22 = 4.5 + .086 = 4.586 What will be the change in pH if 0.01 M [H+] were added to the system? pH =4.5 + log 0.09/0.11 = 4.5 + log 0.818 = 4.5-0.087 = 4.413 So the buffering capacity of a weak acid-conjugate base system is very good near the pKa of the acid Best buffering system is at +/- one pH unit above or below pKa of a weak acid Salivary Buffering-Bicarbonate and pH CO2 + H2O H2CO3 HCO-3 + H + In the oral cavity salivary conc. Of H2CO3 remains essentially constant at 1.3 mM but pH and bicarbonate concentrations do change: 1.When acid is produced within the dental plaque, the reaction is driven to the left and mouth being an open system, CO2 escapes and acid is neutralized and thus saliva protects teeth from decay assuming there is enough bicarbonate present in the plaque. Conversion of H2CO3 to CO2 and water requires carbonic anhydrase VI which is produced by acinar cells of parotid gland and appears to form part the tooth pellicle. Importance of salivary pH • The normal salivary pH is ~6.3 because of salivary bicarbonate. • H2CO3 HCO3-1 + H+ • pKa=6.1 • pH = pKa + log [HCO-13]/[H2CO3] • After meals salivary concentration of HCO3 is increased from ~2mM to 30 mM. Using these values pH at 2 mM HCO-13 will be ~6.29 and at 30 mM HCO3, it should be ~7.46. Variation of pH with bicarbonate concentration Learning Objectives Acids and Bases • Know the definitions of strong and weak acids and bases? • Know how to calculate pH and pKa. • Know the use of Henderson-Hasselbalch equation. • Know the conditions for good buffering action; which are good buffers? • Understand salivary buffering and oral health • Do the problems at the end of this lecture. Problem Set Please do these calculations • A pH of 2.5 means that the [H+] is---------------• For an acetate buffer at pH 4.9, what will be the concentration of acetate ion if the concentration of acetic acid is 0.06 M (pKa of acetic acid is 4.8). • Calculate the pKa of lactic acid given that the pH is 5.28 and the concentrations of lactic acid and lactate are 0.01 and 0.06 M respectively. Ans: • 3.16 x10 –3 M • 0.076 M • 4.5





![[H 2 PO 4 - ] = 0.800 M.](http://s2.studylib.net/store/data/005623813_1-92875a3e2acb84ddbb79ead23a1c6630-300x300.png)