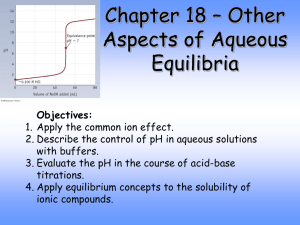
pH of Salt Solutions and Buffers
advertisement

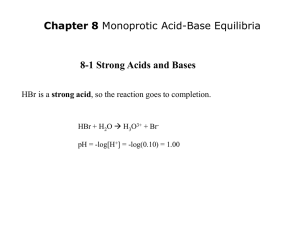
I. Acid-Base Properties of Salts A) Neutral Salts 1) Salt = ionic compound = one that completely ionizes in water 2) Cations of strong bases have no effect on pH 1) Na+, K+, etc… 2) These cations have no affinity for OH- in water 3) Anions of strong acids have no effect on pH 1) Cl-, NO3-, etc… 2) These anions have no affinity for H+ in water 4) Solutions of these combined ion salts have pH = 7.00 B. Basic Salts 1) Salts containing the conjugate base of a weak acid produce basic solutions 2) The conjugate base must be strong if the acid is weak, so it must have a strong affinity for H+, which will affect the pH of a solution 3) Sodium Acetate Example NaC2H3O2 Na+ + C2H3O2[HC2 H3O2 ][OH ] C2H3O2- + H2O HC2H3O2 + OHK ??? b - [C2 H3O2 ] 4) For any weak acid and its conjugate base, Ka x Kb = KW Kb = KW / Ka = 1 x 10-14 / 1.8 x 10-5 = 5.6 x 10-10 for acetate C. Acidic Salts 1) Salts having the conjugate acid of a weak base produce acidic solutions 2) Ammonium chloride = NH4Cl NH4+ + ClNH4+ NH3 + H+ 3) Since ammonia is a weak base, ammonium is “strong” acid and will effect pH 4) Highly charged metal ions can also be acidic a) AlCl3 + 6 H2O Al(H2O)63+ + 3 Clb) Al(H2O)63+ + H2O Al(H2O)5(OH)2+ + H3O+ c) The higher the metal’s charge the more acidic Example: AlCl3 Ka = 1.4 x 10-5 II. Salt Solution Procedure A. Add ~0.5 g of eight different salts to 25 ml water and test with pH paper 1) Use “pH = 7” water and graduated cylinder 2) Don’t have to be very careful with amount; just seeing if acidic/basic/neutral 3) Hydrolysis Reaction = reaction with water (neutral salt: no hydrolysis) NaF: F- + H2O -------> HF + OH- (pH is basic) B. Carefully add 0.500 g of NH4Cl and NaC2H3O2 to 25 ml water; test with pH meter 1) Use “pH = 7” water and Volumetric Flask 2) Need to know mass to 3 decimal places and pH to 2 decimal places 3) Use ICE tables to calculate theoretical pH of your solutions III. Buffers A. A Buffer is a solution containing a weak acid and the salt of its conjugate base (or a weak base and the salt of its conjugate acid) which resist changes to its pH. 1) Example: Blood stays at pH = 7.4, even when various reactions in the body produce much H+ and OH2) The buffer system in blood is H2CO3/HCO33) Buffer Capacity = amount of H+/OH- a buffer can absorb with only a small pH change B. How a Buffer Works 1) Buffers have a large concentration of both HA and A2) When OH- is added, it reacts with HA and is replaced in solution by A3) When we add H+, it is used up by reaction with A- and replaced in solution by HA 4) HA H+ + A- [H ][A- ] [HA] Ka [H ] K a [HA] [A- ] pH depends on [H ], which depends on C. The Henderson-Hasselbalch Equation for Buffers: [HA] [A- ] [A ] pH pK a log [HA] a) We can use this simple equation instead of doing the longer equilibrium problem approach for most buffer problems b) Any concentration of buffer with the same [A-]/[HA] ratio will have the same pH c) The Henderson-Hasselbalch Equation assumes that [A-] and [HA] are the same as [A-]0 and [HA]0. If we keep the buffer concentration high, this is a valid approximation. IV. Procedure and Calculations for Buffers A. Make 50ml of a buffer with pH = 5.00 from 1.00 M Acetic Acid and 5.00 g of Sodium Acetate 1) Example calculation for pH = 5.20 2) Ka = 1.8 x 10-5 so pKa = 4.74 [A ] [A ] 5.20 4.74 log pH pKa log [HA] [HA] [A ] [A ] 100.46 2.88 0.46 log [HA] [HA] 1mol 0.061mol 0.061molNaC2 H 3O 2 (5.00g) 1.22M 0.050L 82.034g [A ] 1.22M 2.88 [HA] 0.424M [HA] [HA] M V (0.424M)(5 0ml) V1 2 2 21.2ml M1 1.00M 3) To make buffer: Add 5.00g Sodium Acetate and 21.2ml 1.00M Acetic Acid to a 50ml Volumetric Flask and dilute with water to the mark. B. Test the pH of your buffer with the pH meter, and calculate %Error %Error Experimental - Assigned Assigned x 100% C. Add 10ml of your buffer to a 100ml Volumetric and dilute to the line. Find pH 1) Original buffer: [HA] = 0.424M [A-] = 1.22M 2) Diluted buffer: [HA] = 0.0424M [A-] = 0.122M D. What is the pH when we add 5.00ml of 0.10M HCl to our diluted buffer? 1) 100ml + 5ml = 105ml = new volume (0.122mol/L)(0.100L) = 0.0122mol A(0.0424mol/L)(0.100L) = 0.00424 mol HA 2) HCl will completely dissociate to H+ (0.10mol/L)(0.005L) = 0.0005mol H+ 3) C2H3O2+ H+ HC2H3O2 Initial After H+ 0.0122mol 0.0117mol 0.0005mol 0 0.00424mol 0.00474mol [A ] 0.105L 0.0117mol/ 4.74 log pH pKa log 5.13 /0.105L 0.00474mol [HA] E. What is the pH when we add 5.00ml of 0.10M NaOH to our diluted buffer? 1) 100ml + 5ml = 105ml = new volume (0.122mol/L)(0.100L) = 0.0122mol A(0.0424mol/L)(0.100L) = 0.00424 mol HA 2) NaOH will completely dissociate to OH- (0.10mol/L)(0.005L) = 0.0005mol OH3) HC2H3O2 + OHC2H3O2- Initial After OH- 0.00424mol 0.00374mol 0.0005mol 0 0.0122mol 0.0127mol [A ] 0.105L 0.0127mol/ 4.74 log pH pKa log 5.27 /0.105L 0.00374mol [HA]