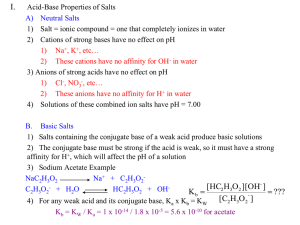
H 3 O + - Valdosta State University
advertisement

Chapter 18 – Other Aspects of Aqueous Equilibria Objectives: 1. Apply the common ion effect. 2. Describe the control of pH in aqueous solutions with buffers. 3. Evaluate the pH in the course of acid-base titrations. 4. Apply equilibrium concepts to the solubility of ionic compounds. The Common Ion Effect • Add Lactate ion – effect? • Le Chatelier’s Principle: Lactic acid will __________________________. • The ionization of an acid or a base is limited by the presence of its ______________________. Preparation of Buffer As shown by an universal indicator: Acetic acid -Acidic solution Sodium acetate -Basic solution Mixing equal amounts of acetic acid and sodium acetate: - Solution with lower hydronium ion concentration than acetic acid. Assume you have a 0.30 M solution of formic acid (HCO2H) and have added enough sodium formate (NaHCO2) to make the solution 0.10 M in the salt. Calculate the pH of the formic acid solution before and after adding sodium formate. HCO2H (aq) + H2O (l) H3O+ (aq) + HCO2-(aq) Buffer • A buffer causes solutions to resist a change in pH when a strong acid or base is added. • Two requirements: - • Buffer is usually prepared from a conjugate acid-base pair: - Common Buffers Buffer • Acetic acid / acetate ion buffer: • Acetic acid, the weak acid, is needed to consume any added hydroxide ion: CH3CO2H(aq) + OH- CH3CO2-(aq) + H2O (l) K = 1.8 x 109 • K is very large – any OH- added will be consumed • Acetate ion, the conjugate base, will consume any added hydronium ion: CH3CO2- + H3O+ CH3CO2H (aq) + H2O (l) K = 5.6 x 104 • K is also large, because H3O+ is a strong acid. What is the pH of a buffer solution composed of 0.50 M formic acid (HCO2H) and 0.70 M sodium formate (NaHCO2)? HCO2H (aq) + H2O (l) H3O+ (aq) + HCO2-(aq) Expressions for Buffer Solutions • Let’s rearrange: Ka = [H3O+][HCO2-] = 1.8 x10-4 [HCO2H] + [H3O+] = [HCO2H] [HCO2-] x Ka [H3O+] = [acid] x Ka [conjugate base] pH = [H3O ] is given by the ratio of the acid and conjugate base concentrations multiplied by the acid ionization constant. This is true for all buffers from weak acid and its conjugate base. Apply –log to each side of the equation: pKa + log [conjugate base] [acid] Henderson-Hasselbalch Equation pH = pKa + log [conjugate base] [acid] • The resulting pH of the buffer is determined primarily by ____________________(or _____) and it is adjusted by varying the _______________ ratio (relative number of moles). • Diluting a buffer will _____________its pH. • When the concentrations of acid and conjugate base are the same, the log of ________ = _________ • If acid = conjugate base ; pH ________ • If conjugate base > acid ; pH _________ • If acid > conjugate base ; pH _________ What is the pH of a buffer solution composed of 0.50 M formic acid (HCO2H) and 0.70 M sodium formate (NaHCO2)? pH = pKa + log [conjugate base] [acid] HCO2H (aq) + H2O (l) H3O+ (aq) + HCO2-(aq) Buffer • To be useful: – pH control: choose a weak acid with ____ ___________________________ – Buffer capacity: Concentration of buffer should be high enough to ____________ ______________________. Buffers are usually prepared as ________________ solutions. Describe how to prepare a buffer solution from NaH2PO4 and Na2HPO4 to have a pH of 7.5. Benzoic acid (C6H5CO2H, 2.0 g) and sodium benzoate (C6H5CO2-, 2.0 g) are dissolved in enough water to make 1.0 L of solution. Calculate pH of the solution using the Henderson-Hasselbalch equation. Calculate the pH of 0.5 L of buffer solution composed of 0.50 M formic acid and 0.70 M sodium formate before and after adding 10.0 mL of 1.0 M HCl. 1) Find the amount of acid formed when HCl reacts with conjugate base. 2) Calculate [H3O+] for the buffer 3) Convert [H3O+] to pH HCO2H (aq) + H2O (l) H3O+ (aq) + HCO2-(aq) Biological Buffer Systems • HPO42- / H2PO4• HCO3-/H2CO3 • Read book p. 862 ed 6. (p. 822 ed 7) Titration and pH Titration of a Strong Acid with a Strong Base • Initial pH (depending on strong acid concentration) – very acidic • As NaOH is added, pH increases very slowly until the equivalence point. • Equivalence point – same number of moles of acid and base • H3O+ + OHH2O • = neutral pH = _______ • As more NaOH is added, pH becomes basic. The pH of the equivalence point in an acidbase titration is the mid-point in the vertical portion of the pH vs volume of titrant curve. Strong-acid/strong-base titration : pH =____ Titration of a Weak Acid with a Strong Base • The initial pH depends on the Ka of the weak acid and the [acid]. • As NaOH is added the pH increases and the conjugate base of the acid is form. • A buffer is generated! • At halfway point of the titration the [acid]=[conjugate base] • pH = __________ • At the equivalence point the solution contains only the conjugate base salt since all acid and base reacted producing the salt. • At eq. pt: The pH depends on ______ of the conjugate base and [conjugate base]. What is the pH of the solution when 35.0 mL of 0.1 M NaOH has been added to 100 mL of 0.1 M acetic acid? 1) Find the [acid] remaining and the [conjugate base] formed after adding NaOH. 2) Find pH for the buffer generated. CH3CO2H (aq) + H2O (l) CH3CO2H (aq) + OH- (aq) H3O+ (aq) + CH3CO2-(aq) H2O (aq) + CH3CO2- (aq) Titration of a Weak Base with a Strong Acid • The initial pH depends on the Kb of the weak base and the [base]. • As HCl is added the pH decreases and the conjugate acid of the base is form. • A buffer is generated! • At halfway point of the titration the [base]=[conjugate acid] • pH = _________________ • At the equivalence point the solution contains only the conjugate acid salt since all base and acid reacted producing the salt. • The solution is __________. Titration of a Weak Diprotic Acid with a Strong Base • Curve shows two inflection points. • First produces a weak base • Second produces a stronger base. If you require 36.78 mL of 0.0105 M HCl to reach the equivalence point in the titration of 25.0 mL of aqueous ammonia. What was the concentration of NH3 in the original ammonia solution? What is the pH of the solution at the equivalence point? NH3 + H3O+ NH4+ + H2O Indicators • An organic compound that is itself a __________________. • The acid form of the compound has one color and the conjugate base another. Indicators • Indicator needs to be chosen so that it changes color at a pH closer to the anticipated equivalence point. Solubility of Salts • • • • Precipitation reaction: CaCl2(aq) + Na2CO3 (aq) CaCO3(s) + 2 NaCl (aq) Insoluble salt in solvent: AgBr(s) Ag+ + BrKsp – Solubility product constant Is an equilibrium constant K = [Ag+][Br-] = Ksp -When the product of the Ksp = concentrations is larger than Ksp the salt will precipitate! Solubility – quantity present in some volume of a saturated solution (gr/L, etc). Insoluble Salts Identify as soluble or insoluble: Pb(NO3)2 Fe(OH)3 ZnCl2 CuS ZnSO4 (NH4)2CO3 Calculate the Ksp value for CaF2 if the concentration of calcium ions in solution (solubility) is 2.4 x 10-4 mol/L CaF2 Ca2+ + 2 F- Knowing the Ksp for MgF2 is 5.2 x 10-11, what is the solubility in moles/L? MgF2 Mg2+ + 2 F- Common Ion Effect Adding an ion “common” to an equilibrium causes the equilibrium to ___________________________. Insoluble Salts Separation of Ions What amount of Cl- is required to precipitate the Hg2Cl2 from a Hg22+ solution of 0.01 M concentration. A solution contains 0.020 M Ag+ and Pb2+. Add CrO42- to precipitate red Ag2CrO4 and yellow PbCrO4. Which precipitates first? Ksp for Ag2CrO4 = 9.0 x 10-12 Ksp for PbCrO4 = 1.8 x 10-14 Solution: The substance whose Ksp is first exceeded precipitates first. Remember • Go over all the contents of your textbook. • Practice with examples and with problems at the end of the chapter. • Practice with OWL tutor. • Work on your OWL assignment for Chapter 18.