Precipitate Reactions
advertisement

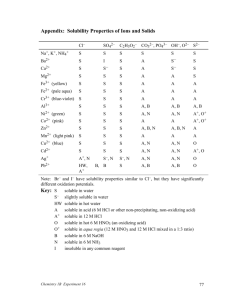
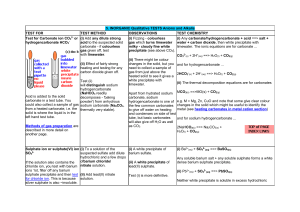
Mix the following solutions in pairs Write down the solution pairs and record your results Potassium Iodide Barium Nitrate Lead Nitrate When finished, try Silver Nitrate with Potassium Iodide. Precipitate Reactions Precipitates Solubility rules Using the rules of solubility Reaction of PbNO3 and KI Lead II Nitrate and Potassium Iodide Both are strong electrolytes That means they completely dissociate into ions Adding the solutions together will create something new… A chemical change the evidence is the precipitate. Look closer at Lead (II) Nitrate Strong Electrolyte Ions completely dissociate into: Pb2+ ions Nitrate NO3 - Ions Lead Pb (NO)3 The KI does the same thing Posassium K+ ions and Iodide I- ions form. They are floating around in the water. (an aqueous solution) When the lead and the iodide ions come into contact, they form a precipitate The precipitate falls to the bottom, spectator ions are left in solution The rules can be broken into 4 categories. 1 soluble no exceptions 2 soluble some exceptions 3 not soluble some exceptions 4 not soluble few exceptions Solubility Rules: Always Always Soluble 1. Always Soluble: Li+, Na+,K+, NH4+ Group 1A(Alkali Metals) and ammonium compounds are soluble. C2H3O2-, NO3-,ClO3-, ClO4 Acetates, Nitrates, Chlorates, Perchlorates are all soluble. The solubility rules: Usually soluble Cl-, Br-, I- Most chlorides, bromides and Iodides are soluble • F- Most fluorides are soluble • SO42- Most Sulfates are soluble Exceptions AP/H (Ag) Silver: AgCl, AgBr, AgI (Pb) Lead (II) PbCl2, PbBr2, PbI2 (Hg) Mercury (I): Hg2Cl2, Hg2Br2, Hg2I2 Exceptions CBS-PM Calcium, Barium, Strontium, Lead, Magnesium MgF2, CaF2, SrF2, BaF2 Lead (II) PbF2 Exceptions CBS/PBS Calcium, Strontium, Barium, Lead (II) CaSO4 PbSO4 SrSO4 BaSO4 The solubility rules: Usually NOT Soluble O2-, OHMost hydroxides and oxides are insoluble Exceptions: CBS CaO, Ca(OH)2 SrO, Sr(OH)2 BaO, Ba (OH)2 And the ‘always group’ of Alkali metals and Ammonium The solubility rules: Insoluble Ions Not soluble CO32- PO43- SO32- S2- C2O42- CrO42- Exceptions: The things that are always soluble. Group 1A (Alkali Metals) Li2CO3, Na2CO3… etc Ammonium compounds (NH4)2CO3 Types of reactions Remember net ionic equations? Showing everything in the reaction vessel (beaker, well plate, test tube… Sometimes parts of the equation do nothing but sit and watch. Spectators Complete ionic vs. net ionic Sodium sulfate + Barium Chloride react to form Solid barium sulfate and Sodium chloride 2 Na+ (aq) + SO42- (aq) + Ba2+ (aq) + 2 Cl- (aq) BaSO4 (s) + 2 Na+ (aq) + 2 Cl- (aq) NaCl is a strong electrolyte, so it does not combine to form a solid Stoichiometry in Aqueous Reactions What volume of 0.200 M copper (II) sulfate is required to react with 50.0mL if 0.100 M NaOH? 1) Write the net ionic equation Cu 2+ (aq) + 2 OH- (aq) Cu(OH)2 (s) What volume of 0.200 M copper (II) sulfate is required to react with 50.0mL if 0.100 M NaOH? find the moles of each reactant needed: nOH- = nCu2+ =