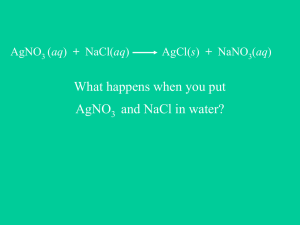Double Replacement Reactions
advertisement
Double Replacement Reactions How do we know if these happen? Double Replacement Reaction 2 KI(aq) + Pb(NO3)2(aq) 2 KNO3(aq) + PbI2(s) Double Replacement Reactions • Two types of Double Replacement Reactions: 1. Formation of an insoluble precipitate • NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s) 2. Formation of a gas • • Acid + salt → gas + another salt H2SO4(aq) + Na2S(aq) → H2S(g) + Na2SO4(aq) Solubility Rules • All sodium, potassium, and ammonium salts are soluble • All acetate, nitrate, and chlorate salts are soluble • All halide salts (F, Cl, Br, I) are soluble – Except lead (II), silver, and mercury (I) • All sulfates are soluble – Except calcium, barium, lead (II), and silver • All carbonates, phosphates, and sulfides are INSOLUBLE – Except sodium, potassium, ammonium Ionic Equations • Since ionic compounds (salts) exist as ions in solution, a precipitation reaction can be shown as ions: – NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s) – Na+(aq) + Cl-(aq) + Ag+(aq) + NO3-(aq) → Na+(aq) + NO3-(aq) +AgCl(s) • This is the ionic equation for the reaction – Be careful with coefficients and subscripts from balanced formula equations Net Ionic Equations • Na+(aq) + Cl-(aq) + Ag+(aq) + NO3-(aq) → Na+(aq) + NO3-(aq) +AgCl(s) • Na+ and NO3- ions are on both side, so they can be cancelled: – Cl-(aq) + Ag+(aq) → AgCl(s) • Na+ and NO3- are spectator ions • They are soluble on both sides of the ionic equation • The net ionic equation shows the reaction without the spectator ions – The net ionic equation shows which ions react and how the reaction occurs For the following sets of reactants… • Predict if a reaction will occur, based on solubility rules • Write a balanced formula equation, if the reaction occurs • Write a balanced ionic equation, if the reaction occurs • Write a balanced net ionic equation, if the reaction occurs Double Replacement Reactions 1. 2. 3. 4. 5. 6. 7. Potassium chromate + silver nitrate Sodium hydroxide + iron (III) chloride Sodium nitrate + calcium chloride Lead (II) acetate + potassium sulfate Silver nitrate + magnesium sulfate Potassium hydroxide + nitric acid Sodium acetate + potassium carbonate