Oxidation States and Criss Cross Method
advertisement
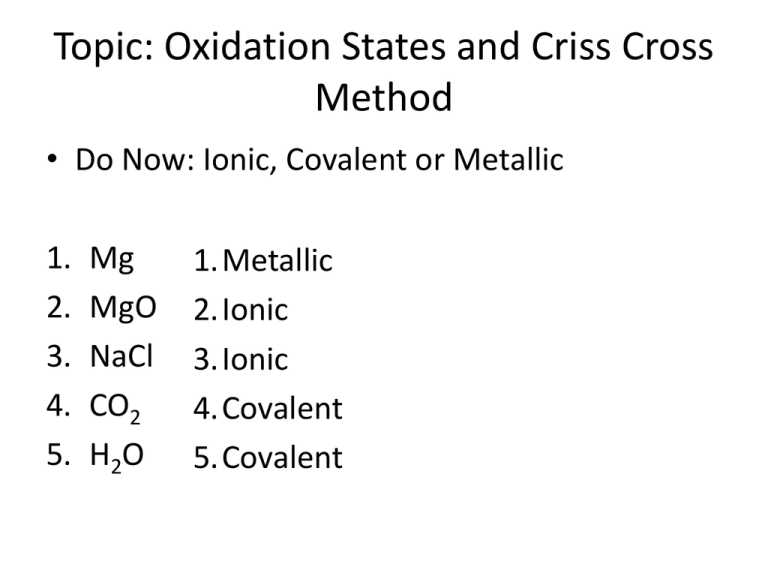
Topic: Oxidation States and Criss Cross Method • Do Now: Ionic, Covalent or Metallic 1. 2. 3. 4. 5. Mg MgO NaCl CO2 H2O 1.Metallic 2.Ionic 3.Ionic 4.Covalent 5.Covalent Ionic bond is a bond between: positive ion and negative ion • Metals: lose valence electrons –form cation (+ ion) • Nonmetals: gain electrons –form anion (- ion) • Metals lose their valence electrons and nonmetals gain IONIC BONDING • Ionic compounds are electrically neutral overall 1 Ca = +2 x 1 = +2 2 Cl = -1 x 2 = -2 2 K = +1 x 2 = +2 1 S = -2 x 1 = -2 2 Cr = +3 x 2 = +6 3 O = -2 x 3 = -6 Assigning Oxidation States • You can look at your periodic table. It’s listed in the topic right corner. • What do you do if there is more then one?! What to do if more then 1 oxidation state • V2S5 –Nonmetal = top number –So S would be -2 +5 -2 –V2S5 ____ +5 x 2 = +10 -2 x 5 = -10 • Then determine the oxidation state of the metal that will give you the same number but positive You try… +2 O= ____ -2 • FeO Fe=____ -2 +3 O= ____ • Fe2O3 Fe=____ Criss-Cross Method Mg+2 and Cl-1 Crisscross and Drop Mg1Cl2 but if subscript is 1, forget it! MgCl2 means 1 Mg+2 and 2 Cl-1 NOTE: The formula has to be in the simplest whole number ratio (this is called the empirical formular Be2O2 = BeO Al3N3 = AlN Try a few formulas: • • • • • • • Ca+2 + Cl-1 Na+1 + O-2 Cs+1 + S-2 Al+3 + Cl-1 Al+3 + Se-2 Mg+2 + F-1 Ca+2 + S-2 CaCl2 Na2O Cs2S AlCl3 Al2Se3 MgF2 CaS