Ionic Bonding Model Cards
advertisement

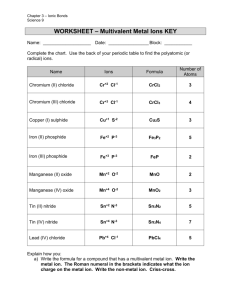
Ionic Bonding Puzzle Lab In this activity you will create models of ionic compounds and identify the chemical formula of the molecules you have created. Procedures 1. Cut the ion models apart carefully. 2. Lay all of them out on the table according to the group number they belong to on the periodic table. 3. Notice the shape and charge of each ion model in each group. Think about why some have “outies” and some have “innies”. 4. What happens when you put any +1 and -1 ion model together? It shows a balanced ionic molecule by creating a perfect rectangle. 5. Look at #1 on the data sheet. Pull one of each ion model for the elements listed. 6. In the first box, write the metal ion name from the model, then write the non-metal ion name under it. 7. Write the ion symbol and charge from each of the models in the second box. 8. Write out only the charge of each ion with a + or – sign in front of the number in the 3rd box. 9. Fit the ion model pieces together. If they do not make a perfect rectangle, then add another piece with the same names until you get a perfect rectangle. 10. In the 4th box, calculate how many of each ion is needed to balance the molecule out to a zero charge. (How many of each made a perfect rectangle?) 11. In the last box, write out the chemical formula for the molecule using symbols and subscripts. 12. Repeat steps 5-11 to finish the data sheet for all ionic bonds. Al+3 Aluminum Al+3 Aluminum F-1 Fluoride Na+1 Sodium F-1 Fluoride K+1 Potassium F-1 Fluoride K+1 Potassium Cl-1 Chloride K+1 Potassium Cl-1 Chloride N-3 Nitride P-3 Phosphide Ca+2 Calcium Cl-1 Chloride Br-1 Bromide Ca+2 Calcium Ca+2 Copper P-3 Phosphide Na+1 Sodium O-2 Oxide Mg+2 Magnesium Li+1 Lithium S-2 Sulfide I-1 Iodide Ti+4 Titanium (IV) O-2 Oxide Na+1 Sodium O-2 Oxide Na+1 Sodium I-1 Iodide