Unit 2 review worksheet
advertisement

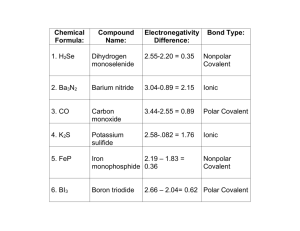
Unit 2 Review Worksheet Ch. 11.1, 11.2, 12.1, 12.3, 7, and 4 Name: Block: Ch. 11.1 - 11.2 - Atoms, Energy and Models of the Atom 1. What is electromagnetic radiation? 2. Draw and label the electromagnetic spectrum below with at least 5 different types of waves. High Energy __________________________________________ Low Energy 3. Define: - photon: - wavelength: - frequency: 4. How are valence electrons different from core electrons? Which are more important during a chemical reaction and why? 5. Describe what happens to the number of valence electrons as you move: - across a period: - down a group: 6. Where do the colors come from in the colored flame demo? Why don’t the colors blend together when you mix two different salt solutions? 1 12.1 & 12.3 - Characteristics of Chemical Bonds; Lewis Structures 1. Explain using the phrase “Noble Gas Envy” why: - groups 1-3 form cations: - groups 5-7 form anions: - group 4 can form either cations or anions: - the calcium ion is Ca2+: - the phosphide ion is P3-: 2. What is an ionic bond? Give 3 examples of molecules with ionic bonds. Explain how you could identify ionic bonding in a substance just by knowing its chemical formula. 3. What is a covalent bond? Give 3 examples of molecules with covalent bonds. Explain how you could identify covalent bonding in a substance just by knowing its chemical formula. a. What is a polar covalent bond? Give an example. b. What is a network covalent bond? Give an example of a network covalent substance. 4. Would an ionic compound such as NaCl conduct electricity when dissolved in water? Explain why or why not. 2 6. Based on electronegativity differences, identify the following bonds as: ionic, covalent or polar covalent a. Cl---Cl b. H---Cl d. Na---Cl 7. What is a polyatomic ion? Give an example. How is a polyatomic ion different than simple cations or anions? 8. Complete this sentence: When drawing the Lewis structure for an uncharged , draw the element symbol surrounded by one dot for every electron. When drawing the Lewis structure of a cation, the element symbol will be surrounded by (give the exact number) electrons, enclosed in brackets and with the shown at the top right. When drawing the Lewis structure of an anion, the element symbol will always be surrounded by (give the exact number) electrons. 9. Draw Lewis structures for: a. Cs b. O2c. Na3N Ch. 7: Chemical Reactions 1. What is a chemical equation? 2. What is the difference between a chemical and a physical change? 3 3. What are at least 4 evidences of chemical change? 4. In a chemical equation, what are the: - reactants? - products? - subscript? - coefficient? 5. What is a balanced equation? What are the 3 steps to balance a chemical equation? 6. When balancing equations, which can you change - the subscripts, or the coefficients? Why? 7. Balance each equation below: a. ___ Ag2(SO4)3 + ___ AlCl3 -----> ___ AgCl3 + ___ Al2(SO4)3 b. ___ Al + ___ CuSO4 ------> ___ Al2(SO4)3 + ___ Cu c. ___Pb(NO3)2 + ___ NaI ------> ___ PbI2 + ___ NaNO3 8. Write the correct chemical equations for the following reactions. Include the symbols and formula names. a. solid magnesium reacts with aqueous hydrochloric acid to form aqueous magnesium chloride and hydrogen gas b. solid potassium reacts with liquid water to form gaseous hydrogen and aqueous potassium hydroxide 4 Ch. 4: Naming Binary Compounds 1. What is a binary compound? Give 3 examples. 2. What is an ionic compound? Give 3 examples. 3. What is a covalent (molecular) compound? Give 3 examples. 4. What is a chemical formula? In the chemical formula: a. What is the coefficient? What does it tell you? b. What is the subscript? What does it tell you? c. When can you multiply the subscripts? 5. What is the first question you should ask yourself when naming a compound? a. What do you do next if the answer is yes? b. What do you do next if the answer is no? 6. When naming a binary compound, when do you use each suffix? - ide: - ate: - ite: 5 7. When naming acids, when do you use each pre-fix and/or suffix? a. hydro -ic: b. – ic: c. –ous: d. How do you know what the formula would be for an acid containing the anion CO32-? 8. Complete the following table Chemical formula Rb2O Ions present 2 Rb+, O23 Na+, N3- Name Rubidium oxide Compound type (1,2,3) Type 1 Aluminum carbonate Cu2(Cr2O7)3 Type 2 Iridium (V) phosphate TiO2 Co2+, S2Boron tri-iodide XeF6 6 Type 3