The structure and properties o
advertisement
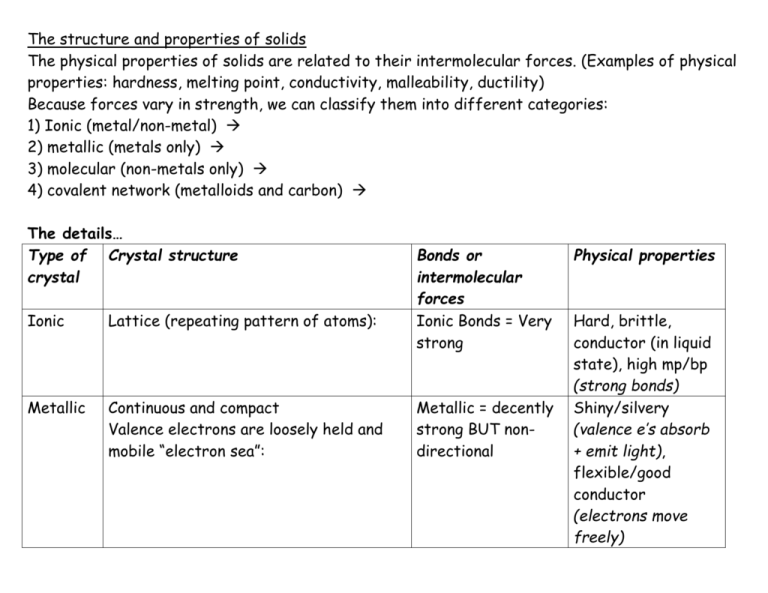
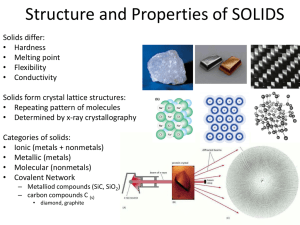
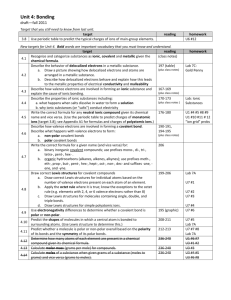
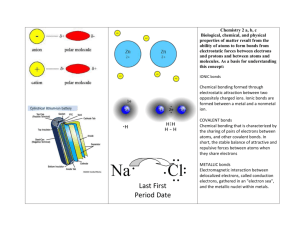
The structure and properties of solids The physical properties of solids are related to their intermolecular forces. (Examples of physical properties: hardness, melting point, conductivity, malleability, ductility) Because forces vary in strength, we can classify them into different categories: 1) Ionic (metal/non-metal) 2) metallic (metals only) 3) molecular (non-metals only) 4) covalent network (metalloids and carbon) The details… Type of Crystal structure crystal Ionic Lattice (repeating pattern of atoms): Metallic Continuous and compact Valence electrons are loosely held and mobile “electron sea”: Bonds or intermolecular forces Ionic Bonds = Very strong Metallic = decently strong BUT nondirectional Physical properties Hard, brittle, conductor (in liquid state), high mp/bp (strong bonds) Shiny/silvery (valence e’s absorb + emit light), flexible/good conductor (electrons move freely) Molecular Lattice like ionic, but packed according to shape and size: Intermolecular: Dipole-dipole London Hydroge = weak!! Low mp/bp, not very hard (weak intermolecular forces), can’t conduct electricity (individual molecules are neutral) Covalent network Covalent bonds: fairly strong… but the strength is amplified by the interlocking covalent network = STRONG! (more so than ionic!) Very hard/very brittle/high melting points (Because MANY bonds are formed to make network), insoluble/don’t conduct electricity (electrons are trapped in the network) Large tetrahedral network with each carbon (or silicon) atom covalently bonded to four other carbon atoms (aka covalent network). Ie: diamonds, quartz: