Predicting Hr using Hess`s law
advertisement

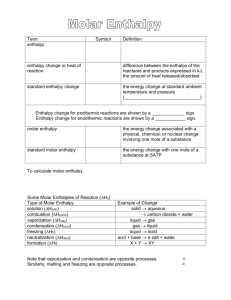
Hess’s Law: The algebraic addition of chemical equations yields a net equation whose enthalpy of reaction is the algebraic sum of the individual enthalpies of reaction. Hnet = Hr 1. 2. If the reaction equation is reversed, the sign of the Hr also reverses. If the coefficients of a reaction equation are changed by multiplying or dividing, then the value of Hr is changed in the same way. 1. 2. 3. 4. 5. Write the balanced chemical equation. Manipulate the given equations so they will add to the net equation. Cancel and Add the remaining reactants and products. Add the component enthalpy changes to obtain the net enthalpy. Determine the molar enthalpy of reaction if required. Practice a) Using the reactions given below, determine the heat of reaction for the equation: 2U(s) + 3O2(g) 2UO3(s) 1) 2UO3(s) 2UO2(s) + O2(g) Hr = 260 kJ 2) U(s) + O2(g) UO2(g) Hr = -1130 kJ b) Determine the molar heat of reaction elements are set as the reference energy state where the potential energy is 0. formation reactions can be endothermic (+H) or exothermic (-H) (p 4-5 data book) simple decomposition is the reverse of a formation reaction so reverse the sign of H thermal stability: ability to resist decomposition the more exothermic a formation reaction, the more endothermic the decomposition= greater thermal stability Compound Molar Enthalpy of Formation kJ/mol aluminum oxide -1675.7 copper (I) oxide -168.6 nitrogen monoxide +91.3 Most stable: aluminum oxide Least stable: nitrogen monoxide To determine the standard enthalpy of a reaction, the sum of the enthalpies of formation of the reactants is subtracted from the sum of the enthalpies of formation of the products. Mathematically, this can be written as: Hnet = nHf(prod) - nHf(react)) watch state of the compound as this affects the molar enthalpy of formation ◦ Ex) H20(l) Ho = -285.8 kJ/mol H20(g) Ho = -241.8 kJ/mol -combustion in bomb calorimeter produces H20(l) Examples: 1. Show the decomposition and formation reactions, with their respective enthalpies for the combustion of propane. 2. Determine the heat of reaction for the reaction of sulfuric acid with magnesium chloride. 3. Determine the enthalpy of reaction for the combustion of 100 g of sucrose. 4. What mass of nitrogen monoxide must react to release 1.00 MJ of energy for the following reaction: 2NO(g) + O2(g) 2NO2(g) 5. The molar enthalpy of combustion of octane is -5047.1kJ/mol. Determine the molar enthalpy of formation of octane.