5.5 Enthalpies of formation
advertisement

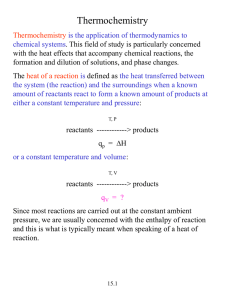
5.5 Enthalpies of formation Homework: pg. 323 #1-2, pg. 324, 4-7 and 8 &9 to submit -A particularly important process used for tabulating thermochemical data is formation of a compound from its constituent elements. -The enthalpy change associated with this process is called Enthalpy of formation (or heat formation) dHf - The subscript f- indicates that the substance has been formed from its constituent elements -The Standard Enthalpy change dHof of a reaction is defined as the enthalpy change where all reactants and products are in their standard state. -The standard state of a substance is its pure form at atmospheric pressure 1atm and temp. 25oC -if elements (in standard state) compound (1 mol in standard state) then dH=dHof -Standard Enthalpies of formation for several compounds are tabulated on pg.320 Nelson For ex: the standard enthalpy of formation for ethanol C2H5OH, is the enthalpy change for the reaction: 2C(graphite) +3H2(g) +1/2O2(g) C2H5OH(l) dHof =-277.7kJ -The stoichiometry of a formation reaction always indicates that one mole of the desired substance is produced. -As a result, standard enthalpies of formation are reported in kJ/mol of the substance being formed -By definition the standard enthalpy of formation of the most stable form of any element is zero, because there is no formation reaction needed when element is already in its standard state. - Sample Ex: 1For which of these reactions at 25oC does the enthalpy change represent a standard enthalpy of formation? For each that does not, what changes are needed to make it an equation whose dH is the enthalpy of formation? a) 2Na(s) +1/2O2(g)Na2O(s) b) 2K(l) + Cl2(g) 2KCl(s) c) C6H12O6 6C(diamond)+6H2(g) +3O2(g) Sample Ex: 2 Using Enthalpies of Formation to calculate Enthalpy of a reaction: We can use Hess’s Law and tabulations of dHof values to calculate standard enthalpy change for any reaction for which we know the dHof values. Target: C3H8(g) +5O2(g) 3CO2(g) + 4H2O(l) dH= ?