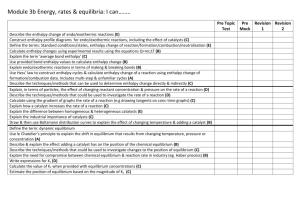
Exam Review - Inquiry Questions 2014
advertisement
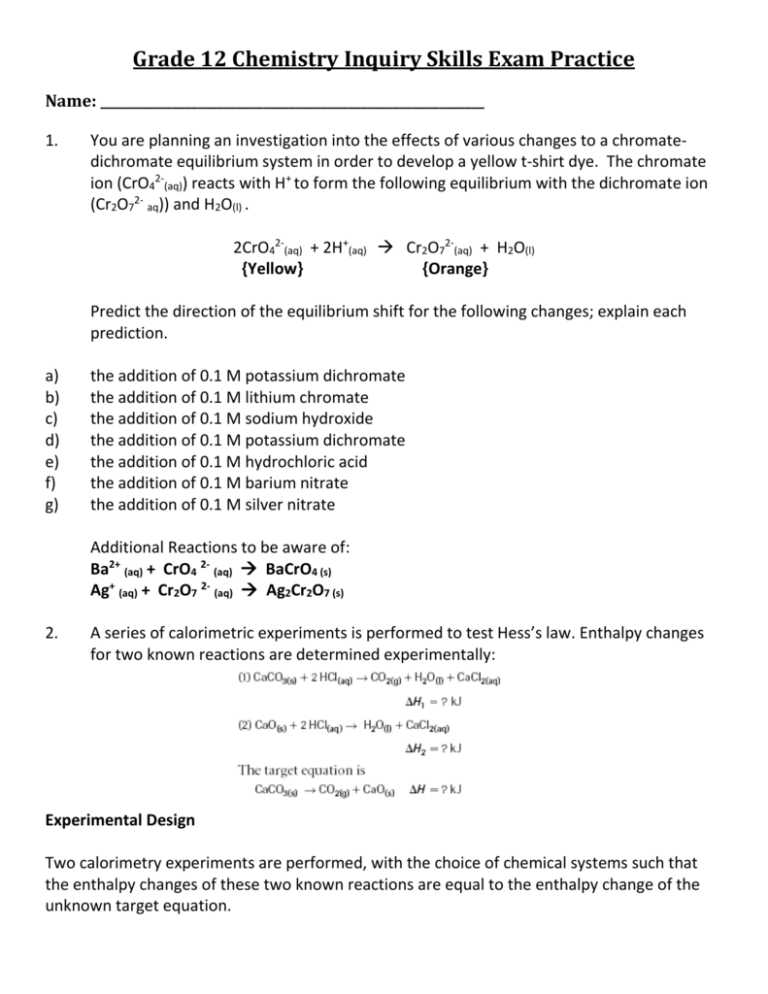
Grade 12 Chemistry Inquiry Skills Exam Practice
Name: ___________________________________________________________
1.
You are planning an investigation into the effects of various changes to a chromatedichromate equilibrium system in order to develop a yellow t-shirt dye. The chromate
ion (CrO42-(aq)) reacts with H+ to form the following equilibrium with the dichromate ion
(Cr2O72- aq)) and H2O(l) .
2CrO42-(aq) + 2H+(aq) Cr2O72-(aq) + H2O(l)
{Yellow}
{Orange}
Predict the direction of the equilibrium shift for the following changes; explain each
prediction.
a)
b)
c)
d)
e)
f)
g)
the addition of 0.1 M potassium dichromate
the addition of 0.1 M lithium chromate
the addition of 0.1 M sodium hydroxide
the addition of 0.1 M potassium dichromate
the addition of 0.1 M hydrochloric acid
the addition of 0.1 M barium nitrate
the addition of 0.1 M silver nitrate
Additional Reactions to be aware of:
Ba2+ (aq) + CrO4 2- (aq) BaCrO4 (s)
Ag+ (aq) + Cr2O7 2- (aq) Ag2Cr2O7 (s)
2.
A series of calorimetric experiments is performed to test Hess’s law. Enthalpy changes
for two known reactions are determined experimentally:
Experimental Design
Two calorimetry experiments are performed, with the choice of chemical systems such that
the enthalpy changes of these two known reactions are equal to the enthalpy change of the
unknown target equation.
a)
Use Hess’s law to show how the two known equations may be added together to yield
the target equation.
Evidence and Analysis
b)
c)
d)
Use the evidence to calculate the molar enthalpy change in each experiment. (Assume
that the specific heat capacity of the acid solution is 4.18 J/g•°C)
Calculate the enthalpy change for the target equation.
Explain the effect that you would expect on the calculated ∆H for reaction (1) if:
i.
ii.
some of the calcium carbonate remained on the weighing paper as it was added
to the acid.
some heat was lost to the air
3.
Explain the following data completely regarding organic compounds in terms of
intermolecular forces involved in each compound.
Compound Boiling Point (OC)
Ethane
-89
Ethanal
21
Ethanol
78
Propanol
97
4.
A local candy company is developing a new apricot-flavoured bubble gum. They have
offered you a one time consulting fee to develop an experimental design for the
production of pentyl butanoate, which is a synthetic ester that smells like apricot.
a)
b)
Create a material list and procedure for the production of the ester. Use any
organic or inorganic compounds that you feel are useful.
Write the reaction including structural diagrams and names for the production of
the ester.