CHEN 354-HW 3
advertisement
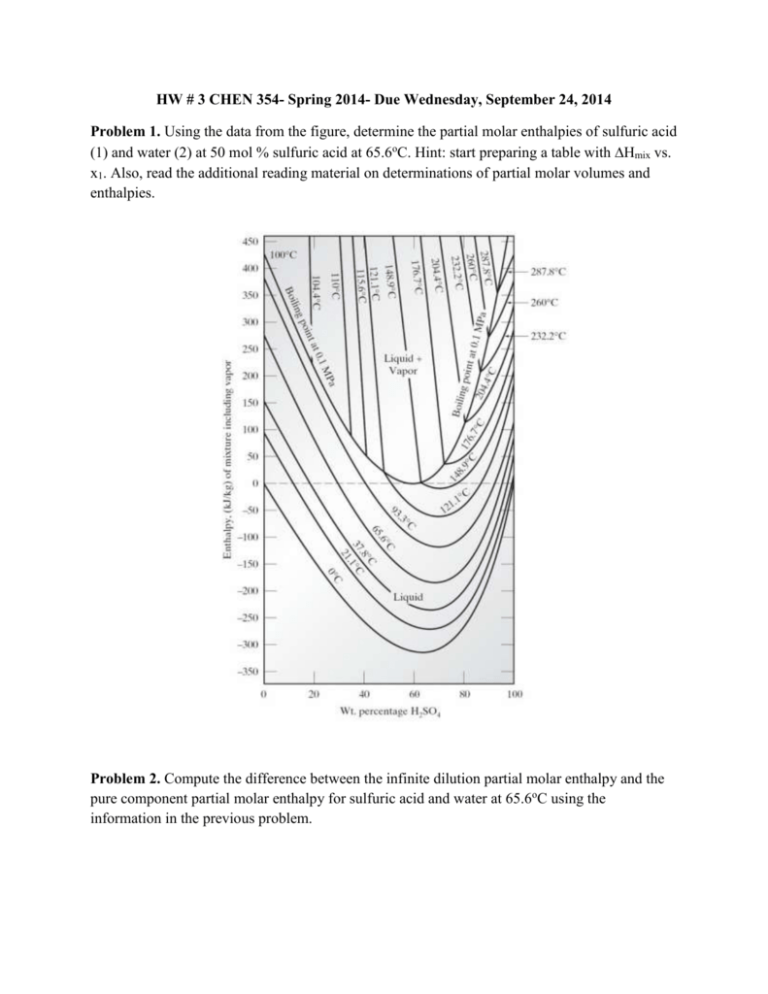
HW # 3 CHEN 354- Spring 2014- Due Wednesday, September 24, 2014 Problem 1. Using the data from the figure, determine the partial molar enthalpies of sulfuric acid (1) and water (2) at 50 mol % sulfuric acid at 65.6oC. Hint: start preparing a table with Hmix vs. x1. Also, read the additional reading material on determinations of partial molar volumes and enthalpies. Problem 2. Compute the difference between the infinite dilution partial molar enthalpy and the pure component partial molar enthalpy for sulfuric acid and water at 65.6oC using the information in the previous problem. Problem 3. A vessel, divided into two parts by a partition, contains 6 mol of N2 gas at 65oC and 30 bar at one side of the partition and 5 mol of Ar at 140oC and 20 bar on the other. If the partition is removed and the gases mix adiabatically and completely, what is the change in entropy? Assume N2 to be an ideal gas with Cv=(5/2)R and Ar to be an ideal gas with Cv=(3/2)R Problem 4. It has been reported that the enthalpies of triethylamine-benzene solutions at 298.15K are given by H xB H B (1 xB ) H EA xB (1 xB ) 1418 482.4(1 2 xB ) 187.4(1 2 xB )3 where xB and xEA are mole fractions of benzene and triethylamine respectively, H is the enthalpy of the mixture and HB and HEA are the pure component enthalpies, in J/mol. a. Develop expressions for H B H B and H EA H EA . b. Compute values of H B H B and H EA H EA at xB = 0.5. c. One mol of a 25mol % benzene mixture is to be mixed with one mole of a 75 mol % benzene mixture at 298.15 K. How much heat must be added or removed for the process to be isothermal?





















