Chapter 8 Basics of Bonding
advertisement

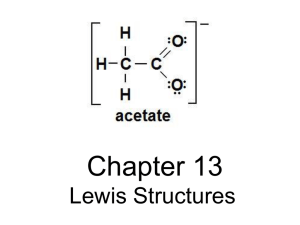
January 10th, 2013 Do Now: 1. How do we differentiate between different bonding types? 2. How are ionic bonds held together? a. Covalent Bonds? b. Metallic Bonds? Chapter 8: Basic Concepts of Chemical Bonding What determines bonding type? How do these bonds give rise to different chemical and physical properties? The Basics of Bonding How do we determine how many electrons we use for bonding purposes? Pre-bonding, how do we represent an atom and its valence electrons? How are the lewis dot symbols for lithium, carbon, and sulfur different? 3, it’s the magic number… oh wait… Why do atoms have only 8 valence electrons represented in the lewis dot symbol? Define octet rule: Which subshells are filled according to the octet rule? Remix! If we represent unbonded atoms with a lewis dot symbol, how do we represent a bonded atom? Compare the lewis structure of an ionic and covalently bonded molecule. How are they different? Why? Diagram both H2O and MgCl2 Subatomically speaking… On a subatomic level, how do 2 hydrogen atoms interact? Based on your knowledge of quantum mechanics, predict the electron density of a molecule of hydrogen gas. Practissimo! Draw the Lewis Structures of the following Molecules: NF3 CH4 HF Compare and contrast these three molecules. How are they similar, how are they different? Double or nothin! How are single and double bonds different? N2 shares how many bonds? Draw it. How is bond length related to the number of electrons shared? How does this bond length affect the reactivity of a molecule of Nitrogen? …therefore… The bond between carbon and oxygen in carbon monoxide is 1.13 angstroms. The carbon and oxygen bond in carbon dioxide is 1.24 angstroms. Without drawing carbon monoxide, does carbon dioxide contain a single, double, or triple bond? Explain your reasoning. North pole, south pole… bond pole? How do we describe bond polarity? How do we determine the bond polarity? Why is an ionic bond not considered or called a polar bond? Electro(n)… affinity? Negativity? How do we define electronegativity? How can we relate electronegativity to electron affinity and ionization energy? Predict the general trend. January 11th, 2013 Do Now: Explain the difference between electronegativity and electron affinity. In the structure ClO2-, which would be the central bonding atom and why? Draw it out! How do we draw lewis structures? Step-by-Step success: 1. Sum all valence electrons for all atoms (how many does each have… add them together!) 2. Write the chemical symbols (highest electronegative element in the middle!) and attach them by single bonds 3. Complete the octets around all elements except central bonded atom. 4. Place any left over valence electrons on central atom even if it breaks the octet rule. 5. Not enough valence electrons on the central atom? Try multiple bonds. Practice Lets try one together… PCl3 You try! How many valence electrons are in the compound CH2Cl2 Draw it! Draw HCN in step form. Write out the step instructions in your own words as you draw step by step! Just a few more… Draw the Lewis structures for: NO+ C2H4 BrO3- Continuing on… Draw the Lewis Structure for CO2 Is this the only possible structure that fulfills the octet rule? How do we determine which structure is most imporant? January 14th, 2012 Do Now: Have out your homework and compare your answers to the key up front. …rewind. …so remember that time Ms. Daly tried to tell you that the most electronegative element was in the middle…? JUST KIDDING. Other way around. Oh, and remember formal charge? Formal Charge: a test to determine the efficiency of electron distribution of a molecule So now, lets try this again. Formally speaking, for real. Why is calculating formal charge useful? If there are multiple lewis structures in which the octet rules are completed, it determines which structure is the most important. (dominant structure) How do we determine the dominant structure: Formal charge should add up to the charge on a current ion or molecule. Neutral must = 0. **note: dominant structure will produce formal charges of the smallest magnitudes** Generally: Dom. Structure is the one in which atoms have formal charges of zero If negative charge, it generally resides on the most electronegative …its all a lot of words… How do we assign the formal charge: Formal Charge = original number of valence electrons – calculated valence electrons Great. How do we assign electrons: 1. All non-bonding electrons are assigned to the atom on which they are found 2. For any bond, ½ electrons are assigned to each atom. Lets try the cyanide ion together. Draw the Lewis Structure of CN- CAUTION FORMAL CHARGE DOES NOT REPRESENT REAL CHARGE ON ATOM. Praktyk (Afrikaans) There are 3 possible Lewis Structures for the thiocyanate ion, NCS-. Draw all three Determine the formal charge for each atom in each structure. Which is the dominant structure. Why? Repeat the above for Cyanate ion: NCO- How about the ozone. Draw the Ozone Molecule and calculate the formal charge. Which is dominant? How can we define a set of Lewis structures when neither is dominant? Resonance Structures: Equally preferred Lewis structures for the same molecule How many resonance structures are there for NO3- Praktikë (Albanian) Which of these two molecules will have a shorter S-O bond length? SO3 or SO32-? Why? Draw the two equivalent structures for HCO2- How many resonance structures does benzene have? So.. How important was that octet rule anyways? Octet rule exceptions: Three Main Types Molecules and polyatomic ions containing an odd number of electrons Molecules and polyatomic ions in which an atom has fewer than an octet of valence electrons Molecules and polyatomic ions in which an atom has more than octet of valence electrons You're… odd…. Most molecules and polyatomic ions have an even number of valence electrons and can be evenly paired off. Exceptions exist: ClO2 , NO, NO2, O2 Complete pairing of these is impossible. Draw NO You’re not greater than… you’re less than! Fewer than eight valence electrons around an atom in a molecule or polyatomic ion. Most common encountered in compounds containing Boron or Beryllium Draw BF3 Why does a double bonded atom defy chemistry? What does this suggest for the reactivity of this molecule? …Oh, so YOU’RE greater than. 3rd and MOST COMMON exception has more than eight valence electrons. CALLED HYPERVALENT Only formed for central atoms from period 3 and below. Reason being? LARGE SIZE. Phosphorus is large enough to accommodate 5 bonds without being too crowded (generally bonded to smaller atoms: F, Cl, O) ( ممارسةarabic) Draw the Lewis Structures for the following: ICl4 XeF2 Which of the following atoms is never found with more than octet of valence electrons around it: S, C, P, Br. Why? Last but not least. How do you decide which Lewis structure to choose when you have to choose between the octet rule and obtaining the most favorable formal charges? Strength of Covalent Bonds How does the strength of covalent bonds relate to the stability of a molecule? How is the strength of a covalent bond measured? Define bond enthalpy. Say what? How do we represent bond enthalpy? How can we define “atomization”? How can you use the enthalpy of atomization of the hydrocarbon ethane, C2H6 along with the value D(C-H) = 413 kJ/mol to estimate the value of D(C-C) ? Bond Enthalpies and Enthalpies of Reactions How can we use the bond enthalpy to estimate the enthalpy of reaction? Consider this reaction: H-CH3(g) + Cl-Cl(g) Cl-CH3 + H-Cl(g) How do we calculate the enthalpy of the reaction? How does bond enthalpy relate to bond length? Պրակտիկա (Armenian) Using the table provided: Estimate the delta H for the reaction: 2 C2H6 + 7 O2 4 CO2 + 6 H2O Try this: N2H4 N2 + 2 H2