Assignment #15
advertisement
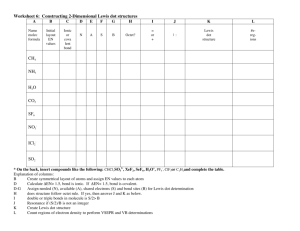
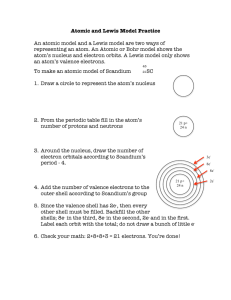
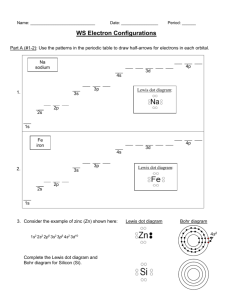
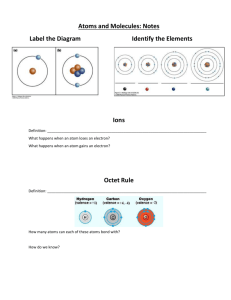
Assignment #15 Lewis Dot Model (Structure) Recall • Quickly write how to draw a Bohr model. Recall • Bohr models detail the energy levels • How can you find the number of energy levels using a periodic table? Lewis Dot • A Lewis Dot Model is an easy way to show how many electrons an atoms has in an outer energy level. Instead of drawing all of the rings as you would with a Bohr Model • Lewis Dot structure shows ONLY the VALENCE ELECTRONS. • How can you find the number of valence electrons on the periodic table? Lets draw the Lewis Dot Model • 1. Write the chemical symbol or abbreviation for the element. For example, Flourine is F • 2. Find the column the element is in of the PTOE. The top of the column has a number. For example, fluorine is 17 • 3. The last number of the column is how many valence electrons the atom has. For example, fluorine has 7 • 4. Now draw that number of dots around the symbol to show how many valence electrons fluorine has. •F Add to your Foldable • Now using your new knowledge add to your foldable! Examples • Please draw the Lewis Dot Model for the following elements. • Carbon Bromine Calcium • Hydrogen Potassium Phosphorous • Chlorine Boron Sulfur • Oxygen Argon Lithium • Silicon Magnesium Aluminum