Lewis structures
advertisement
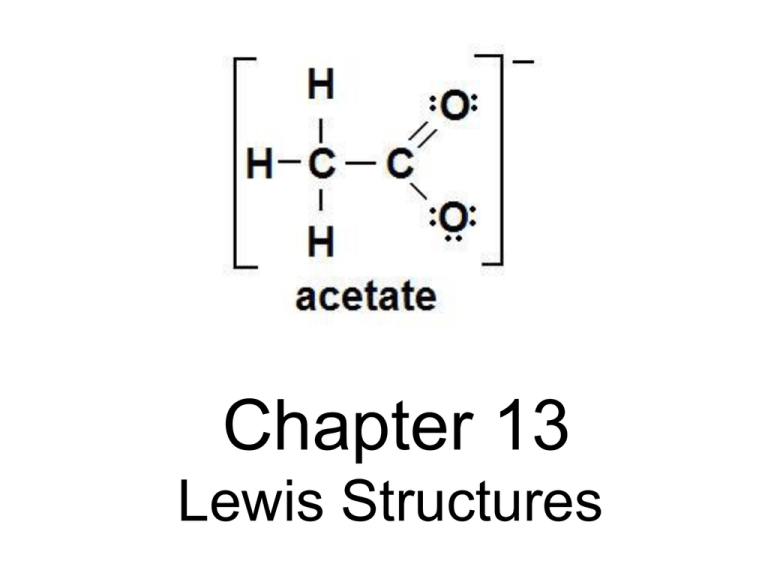
Chapter 13 Lewis Structures Lewis Structures • Lewis structures are diagrams that show the bonding between atoms of a molecule, and the lone pairs of valence electrons in the molecule. Steps for Writing Lewis Structures 1. Count the total number of outer level (valence) electrons. To do this use the Roman numeral from the group number above each element in the periodic table. • PO4 • PO43• PO43+ Steps for Writing Lewis Structures 2. Determine the layout of the molecule. The formula of the molecule will often give you a hint as to its layout. For example in the molecule H3CCH3 there are two carbon atoms in the center with three hydrogen atoms bonded to each carbon. Carbon is always central Hydrogen is never central The element with the lowest electronegativity is central The element which you have the least of is usually central Group 17 elements are usually not central (unless you have no other choice) because they can only form single bonds. • • • • • – After determining the layout of the molecule. Arrange the elements symmetrically around your central atom(s). Steps for Writing Lewis Structures 3. The valence electrons from step 1 are now used to stabilize the atoms. This is done by using shared pairs (bonds) (see Table 1) to attach the atoms to the central atom. Use single bonds for all atoms first. Valence electrons in Lewis Structures Appear as: (1) Shared Pairs (form bonds) — Single bond (one shared pair) Weakest and longest covalent bond ═ Double bond (two shared pairs) Strength and length between single and triple bonds ≡ Triple bond (three shared pairs) Strongest and shortest covalent bond (2) Unshared Pairs (Lone Pairs) xx, ○○, or ●● Steps for Writing Lewis Structures 3. After adding single bonds you may find that the atoms still need more valence electrons to achieve their octets. If more valence electrons are needed use unshared pairs (lone pairs) (see Table 1) around each atom to give the atom an octet (8 valence electrons). There are some exceptions to the octet rule. The most common exception is hydrogen which only requires 2 electrons to fill its outer level and become stable. Valence electrons in Lewis Structures Appear as: (1) Shared Pairs (form bonds) — Single bond (one shared pair) Weakest and longest covalent bond ═ Double bond (two shared pairs) Strength and length between single and triple bonds ≡ Triple bond (three shared pairs) Strongest and shortest covalent bond (2) Unshared Pairs (Lone Pairs) xx, ○○, or ●● Steps for Writing Lewis Structures 3. The valence electrons from step 1 are now used to stabilize the atoms. This is done by using shared pairs (bonds) (see Table 1) to attach the atoms to the central atom. Use single bonds for all atoms first. • After adding single bonds you may find that the atoms still need more valence electrons to achieve their octets. If more valence electrons are needed use unshared pairs (lone pairs) (see Table 1) around each atom to give the atom an octet (8 valence electrons). There are some exceptions to the octet rule. The most common exception is hydrogen which only requires 2 electrons to fill its outer level and become stable. • If you still do not have a stable structure you may try double and triple bonds if C, N, or O is involved in the bond. Steps for Writing Lewis Structures 4. If you cannot write a stable structure for the molecule using rules 1 – 3 add or remove unshared pairs to/from the central atom until you arrive at the desired number of valence electrons determined in step 1. This may give you a structure that appears to be unstable however some molecules can form which do not have stable octets. • Common exceptions to the octet rule: H is stable with 2 valence electrons; B is stable with 6 valence electrons. Writing Lewis Structures CO2 Writing Lewis Structures PO4 3- Writing Lewis Structures H2CO Writing Lewis Structures BrNO Writing Lewis Structures BrNO Steps for Writing Lewis Structures 2. Determine the layout of the molecule. The formula of the molecule will often give you a hint as to its layout. For example in the molecule H3CCH3 there are two carbon atoms in the center with three hydrogen atoms bonded to each carbon. Carbon is always central Hydrogen is never central The element with the lowest electronegativity is central The element which you have the least of is usually central Group 17 elements are usually not central (unless you have no other choice) because they can only form single bonds. • • • • • – After determining the layout of the molecule. Arrange the elements symmetrically around your central atom(s). Writing Lewis Structures HCCH Steps for Writing Lewis Structures 2. Determine the layout of the molecule. The formula of the molecule will often give you a hint as to its layout. For example in the molecule H3CCH3 there are two carbon atoms in the center with three hydrogen atoms bonded to each carbon. Carbon is always central Hydrogen is never central The element with the lowest electronegativity is central The element which you have the least of is usually central Group 17 elements are usually not central (unless you have no other choice) because they can only form single bonds. • • • • • – After determining the layout of the molecule. Arrange the elements symmetrically around your central atom(s). Writing Lewis Structures HCCH Writing Lewis Structures HCCH Writing Lewis Structures BeCl2 Steps for Writing Lewis Structures 4. If you cannot write a stable structure for the molecule using rules 1 – 3 add or remove unshared pairs to/from the central atom until you arrive at the desired number of valence electrons determined in step 1. This may give you a structure that appears to be unstable however some molecules can form which do not have stable octets. Homework Lewis Structures Worksheet Homework Lewis Structures Worksheet