How to make a Bohr model!
advertisement

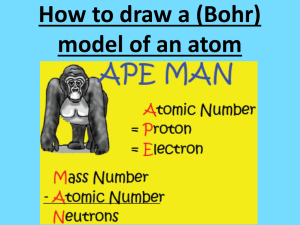
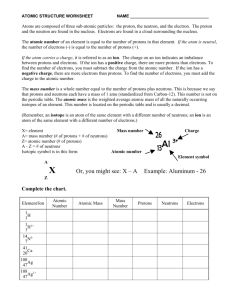
How to make a Bohr model! You can do it! • Choose an element • Look at the atomic number: that tells you the number of protons and electrons • Look at the atomic mass and then round it to the nearest whole number. Now you have the mass number • Subtract the atomic number from the mass number. Now you have the number of neutrons. • At the top of your paper write: MY BOHR MODEL OF ( write the name of the element you’re doing) • Make a key in the corner. Label it: KEY • Write the word protons, then a +, and then the number of protons your element has. (look at the atomic number) • Write the word electrons, then a -, and then the number of electrons your element has. (look at the atomic number) • Write the word neutrons, then a 0 and the number of neutrons your element has. (subtract the atomic number from the mass number) • In the center of your paper draw the number of + you need to have the correct number of protons. • In between the + protons, draw the 0’s to represent the correct number of neutrons you need. • Look in the upper right corner of the element box at the electron numbers. • Draw the correct number of rings around the nucleus. • On the ring closest to the nucleus, make the correct number of – signs for the electrons. (this is the top number in the list; probably 2) • On the next ring, make the correct number of – signs for the electrons. • Continue until you have the correct number of electrons on each ring. • • • • • Double Check! Count the protons. Does it match the atomic number? Count the electrons. Does it match the atomic number? Count the neutrons. Add that number to the number of protons. Does that match the mass number? Do you have protons + and neutrons 0 in the nucleus? Are electrons – on the rings around the nucleus? YOU DID IT!!! You just made a Bohr model! Excellent work! Yay!