Test Study Guide - Waterford Public Schools
advertisement
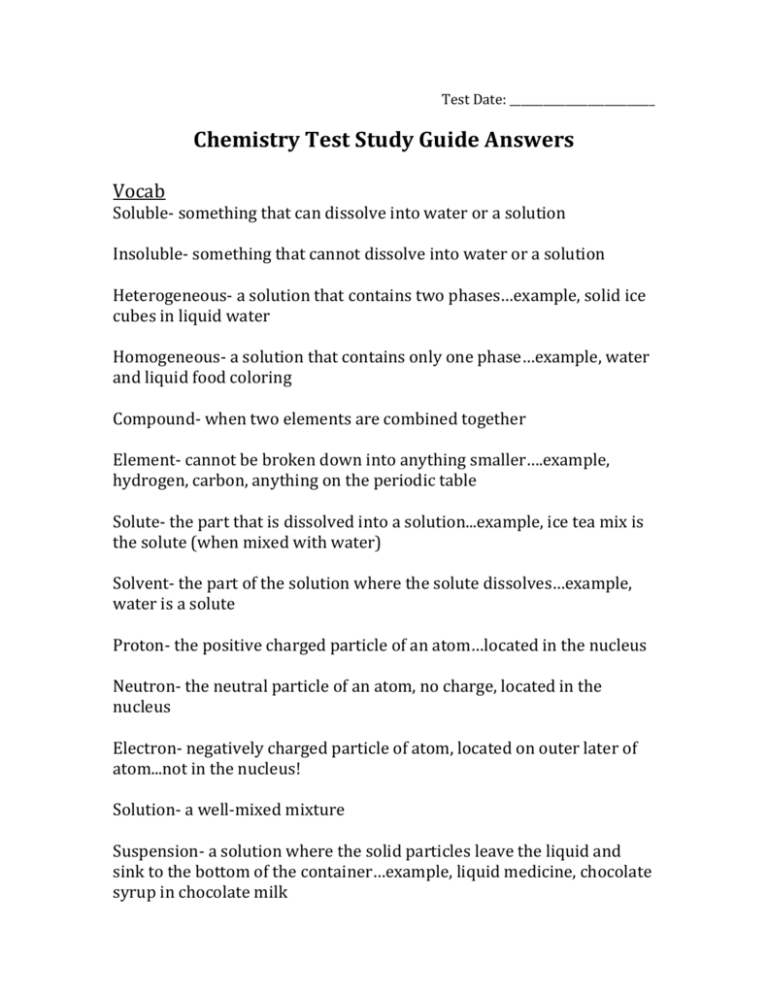
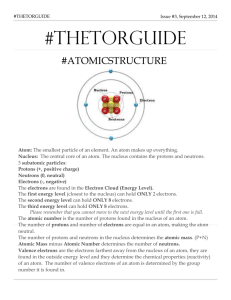
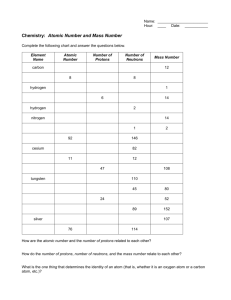
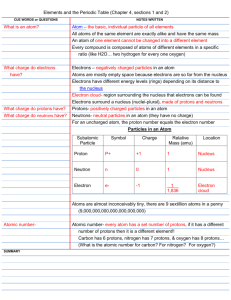
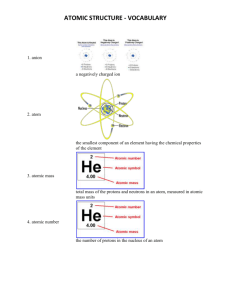
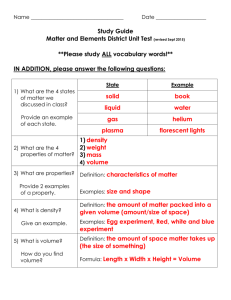
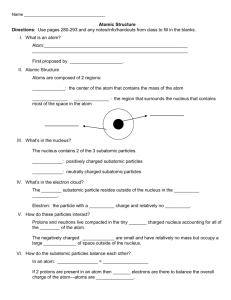
Test Date: __________________________ Chemistry Test Study Guide Answers Vocab Soluble- something that can dissolve into water or a solution Insoluble- something that cannot dissolve into water or a solution Heterogeneous- a solution that contains two phases…example, solid ice cubes in liquid water Homogeneous- a solution that contains only one phase…example, water and liquid food coloring Compound- when two elements are combined together Element- cannot be broken down into anything smaller….example, hydrogen, carbon, anything on the periodic table Solute- the part that is dissolved into a solution...example, ice tea mix is the solute (when mixed with water) Solvent- the part of the solution where the solute dissolves…example, water is a solute Proton- the positive charged particle of an atom…located in the nucleus Neutron- the neutral particle of an atom, no charge, located in the nucleus Electron- negatively charged particle of atom, located on outer later of atom...not in the nucleus! Solution- a well-mixed mixture Suspension- a solution where the solid particles leave the liquid and sink to the bottom of the container…example, liquid medicine, chocolate syrup in chocolate milk Physical and Chemical Changes What is a physical change? A reversible reaction can change back to what it looked like before the reaction. Change how something looks, but not what compound it is made up of. For example, ice melting is a physical change because it is still water, just in a different form. What is a chemical change? An irreversible reactioncannot be changed back to what it was before. You are making a new compound or solution. For example, hard boiling an egg is a chemical change. You cannot un hard boil an egg. The egg was cooked and new compounds were made. Also, baking cookies. You have gooey cookie dough before, then bake it, then have cooked cookies after. This is a chemical change because you cannot unbake those cookies to get back the gooey cookie dough. What is the difference between the two? See above. Example Questions: Label Physical or Chemical Change 1) Scrambling an Egg physical 2) Cooking an Egg chemical 3) Making a new compound chemical 4) Melting Ice to make water physical The Periodic Table How are elements organized? Elements are organized into families. The families (and groupscolumns) have similar chemical properties. They act alike, even though each element is different in its own way. Elements are put in order of their atomic mass (how much space it takes up/weighs) and their atomic number (numbers go in order from left to right….like reading a book) What is a metal? A metal is a shiny object. Metals can be rolled into sheets or wires (very moldable). They can also make electricity and heat. What is a non-metal? A non-metal is very bull and brittle (easily breakable). The cannot make heat or electricity. Be able to draw and label an atom: protons, neutrons, electrons See science notebook for worksheet “Carbon Atom Model”. Should be able to tell me that the electrons are also on the outside of the shell and that the protons and neutrons are in the nucleus (in the middle of the atom). How do you figure out an elements protons, neutrons, and electrons? (Math Challengesee worksheet) Be able to see element given and identify the atomic number, mass, number of protons, neutrons, and electrons. Protons=atomic mass Electrons= atomic mass Neutrons= atomic mass – atomic number