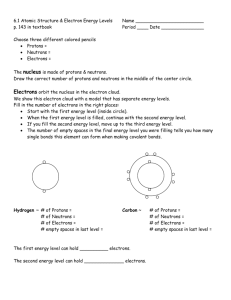
1) According to modern atomic theory, electrons are found where
advertisement

Name _______________________________ Date __________________ SOL PS.3 1. According to modern atomic theory, electrons are found where? 5. Which particles can move from one atom to another during a chemical reaction? A closely attached to the nucleus. B in fixed orbits at well defined distances from the nucleus. C in totally random and varying orbits around the nucleus. D occupying unspecified regions around the nucleus based on how much energy each electron has. A B C D protons neutrinos neutrons electrons 6. Which particle has a negative charge? 2. The nucleus of an atom is composed of what particles? A B C D A B C D 7. The earliest atomic theory held that atoms were: protons and neutrons. protons and electrons. neutrons and isotopes. electrons and neutrons. 3. The atomic number of an element is equivalent to its: A B C D number of valence electrons. number of protons and neutrons. number of protons. number of neutrons. 4. Bohr’s model of the atom proposed that: A electrons move around the nucleus in orbits. B neutrons move around the nucleus. C neutrons do not exist, but are just paired protons and electrons. D the nucleus spins. proton electron neutron negatron A like a pudding with the electrons randomly spread like raisins. B fixed nuclei with the electrons in fixed orbits C like smooth, fixed billiard balls. D a stew with the different subatomic particles randomly floating around. 8. Which of the following statements is true according to current thinking? A an atom is a collection of particles so tightly packed that there is little empty space B the inside of an atom is mostly empty space C the atomic nucleus is very mobile and moves around inside a cloud of electrons D the size of atoms changes over time, pulsating from large to small to large again. _______________________________________________________________________________________________________ Copyright©1999,2000 S.S. Flanagan & D.E. Mott 9 Do not reproduce without permission. 07-15-00