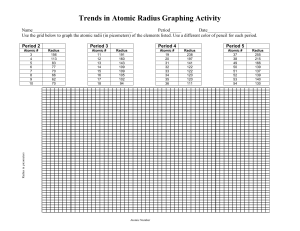
how big an atom is
advertisement

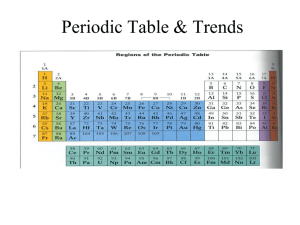
Catalyst 12/1/11 1. How many valence electrons does Argon have? Draw its Lewis dot structures. 2. Draw the Lewis dot structures for the following elements: Ne, Br, Mn, Zn, Ca, K, Ti, Xe, N 3. If an the atom of an element has a mass number of 21 atomic mass units (amu) and has 10 protons. How many neutrons does it have? (Hint: # of neutrons = mass # atomic#) 4. The elements in group 3-12 are called the _______ metals and are part of the ___ block. 5. What are the Group 17 elements called? 6. Which elements in the periodic table are NOT REACTIVE or inert? Agenda 12/1/11 1. 2. 3. 4. 5. 6. 7. Catalyst Announcements Verbal Drill Exploration of The Elements iPad App Atomic Radius Class Practice Exit Slip Homework • Rough Draft of Data and Calculations Section of Lab Report, DUE FRIDAY 12/2/11. Class website: http://mrgchem.weebly.com Announcements • Make up Unit#4 exam today at 7th period or after school. A note will be required if you need to take it after Friday. • Tutoring will be here in Room 22 for the rest of this week. Verbal Drill (2 minutes) • One person speaking at a time (so everyone can hear the question and answer!) • You may only go once • DO NOT CALL OUT IF ITS NOT YOUR TURN. • If you don’t know the answer, have a classmate help you. You still need to say the answer if its your turn! • Class with the most Verbal Drill points will receive 5 points in the Chapter 5 test • P4: 15 pts P5: 13 pts P8: 14 pts P9: 16 pts P10: 18 pts The Elements iPad App Objective SWBAT define atomic radius and describe the atomic radius trend in the periodic table. Quick Review • Valence electrons are the electrons found in the outermost electron shell* and they are the electrons that participate in chemical reactions. (*You know how many valence electrons by looking at the electron shells with the highest principal quantum number.) Atomic Radius Atomic radius is distance between the nucleus and the valence electrons measured in picometers.* (how big an atom is) (1 picometer = 1 x 10-12 meters) *This depends on how it is measured. Copy the words in bold. Atomic Radius Periodic Trends • In general, atomic Radius increases down a group (from up to down) o This is because energy levels are added as you go down the group. • In general, atomic Radius decreases across a period (from left to right) Copy this. Yes, the whole slide. Class Practice 1. As atomic number increases within Group 15 on the Periodic Table, atomic radius • A) decreases, only • B) increases, only • C) decreases, then increases • D) increases, then decreases Class Practice 2. An atom of which element has the largest atomic radius? A) Fe B) Mg C) Si D) Zn 3. Which list of elements from Group 2 on the Periodic Table is arranged in order of increasing atomic radius? A) Be, Mg, Ca B) Ca, Mg, Be C) Ba, Ra, Sr D) Sr, Ra, Ba 4. 5. Magnesium has an atomic radius of 160 pm and Aluminum has an atomic radius of 143 pm. Which of the following is the most likely value for the atomic radius of Sulfur? a. 150 pm b. 190 pm c. 130 pm d. 205 pm 6. Beryllium has an atomic radius of 112 pm and Calcium has an atomic radius of 197 pm. What is the most likely value for the atomic radius of Barium? a. 150 pm b. 160 pm c. 215 pm d. 100 pm 7. Arrange the following elements in order of DECREASING atomic radius: Ar, Ne, He, Xe, Rn. (highest first then lowest) Periodic Table Rap… A little corny, but still entertaining. It might help you remember the properties in the periodic table. Exit Slip On a half a slip of paper, answer the following: 1. Atomic radius increases: 2. Based on their locations on the periodic table, which has a larger atom: Calcium or Zinc? 3. Which has a greater atomic radius: Potassium or Cesium?