File - Grade 8 Science
advertisement
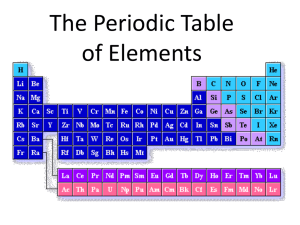
Introduction to Chemistry Part 1 The Unit Organizer 8th grade Science SMS 13-14 NAME 4 BIGGER PICTURE DATE Energy & Matter LAST UNIT /Experience 2 CURRENT CURRENT UNIT UNIT 1 Introduction Chemistry Safety & Scientific Investigation 8 UNIT SCHEDULE 5 Physics studying the chemical & physical properties of matter by describing the atomic structure 8.5 AB The Periodic Table of Elements 8.5 C 6 Describe Identify Translate UNIT 1. How would you compare and contrast the three main subatomic particles found in the atom? Include size, mass, and location. 2. How are elements arranged on the Periodic Table of Elements? 3. How do protons determine an elements identity? How do valence electrons determine an elements chemical properties? identifying & translating RELATIONSHIPS UNIT SELF-TEST QUESTIONS NEXT UNIT /Experience UNIT MAP 9/8 Launch Unit Organizer 9/9 Atomic Structure Time line 9/10 Comparison Table 9/11 Bohr Models/ Atomic Mass Investigation 9/12 Safety & Atoms EOU 9/15 Menu the Periodic Table Patterns 9/16 Stations Lab Properties of elements 9/17 Stations Lab Properties of elements 9/18 NOVA App w DYOD 9/19 Choice Board Activity Assessment 9/22 Valence Electrons & Charges 9/23 Kahoot.it/ Element Riddles QR codes 9/24 Visual Summary 9/25 Tie Up UO 9/26 Periodic Table EOU 7 3 The Unit Organizer 9 NAME DATE Introduction to Chemistry Expanded Unit Map studying the chemical & physical properties of matter by identifying & translating describing The Periodic Table of Elements the atomic structure & naming the 3 Atomic Number Subatomic particles including by is used to Classify Elements their location, charge, & mass; proton is in the nucleus, positive, 1amu The number of protons will determine an elements identity. the neutron is in the nucleus, neutral, 1amu electron is Outside the nucleus, negative, 0amu Electrons on the outermost orbital are called valence electrons. The number of valence electrons will determine the elements reactivity. NEW UNIT SELF-TEST QUESTIONS 10 is the # of protons and is unique for each pure substance. by Metals, Nonmetals & Metalloids Each with physical properties like luster, malleability, conductivity into into Groups Periods with similar physical properties b/c of the # of valence electrons with similar in size b/c they have the same # of orbitals. The Unit Organizer 8th grade Science SMS 13-14 2 5 CURRENT CURRENT UNIT UNIT DATE 3 NEXT UNIT /Experience Introduction Chemistry UNIT MAP 1. How would you compare and contrast the three main subatomic particles found in the atom? Include size, mass, and location. 2. How are elements arranged on the Periodic Table of Elements? 3. How do protons determine an elements identity? How do valence electrons determine an elements chemical properties? 6 UNIT 7 UNIT SCHEDULE 1 BIGGER PICTURE RELATIONSHIPS UNIT SELF-TEST QUESTIONS 8 LAST UNIT /Experience NAME 4 The Unit Organizer 9 Introduction to Chemistry Expanded Unit Map studying the chemical & physical properties of matter by NEW UNIT SELF-TEST QUESTIONS 10 NAME DATE