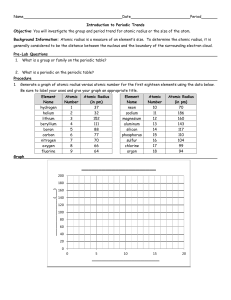
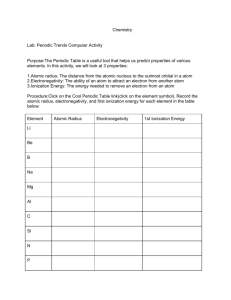
Periodic Table Trends
advertisement

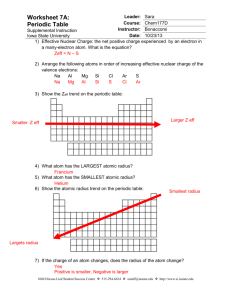
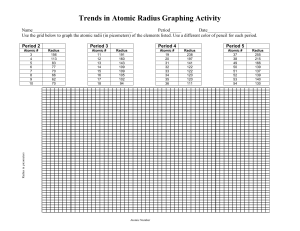
http://educationportal.com/academy/lesson/the-diagonalrelationship-boiling-point-and-metallicproperties.html Metallic character increases downward and increases from right to left across the period. Electrical conductivity increases downward and to the left density generally increases as atomic number increases. http://www.ptable.com/ Atomic radius increases from right to left within a period Atomic radius increases downward within families Energy required to remove an electron. Define ionization energy and how it trends from the following video: http://educationportal.com/academy/lesson/ionizationenergy-trends-among-groups-and-periodsof-the-periodic-table.html Loss of electrons from an atom Examples… Li to Li +1, F to F -1