Unit 2 Overview
advertisement

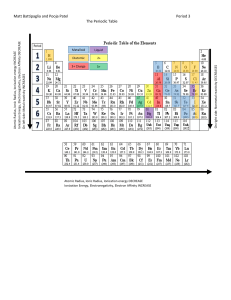
UNIT 2 CHEMISTRY Unit 2 Over View 2.1 The Atom 2.2 The Periodic Table 2.3 Elements 2.4 Chemical Bonding Concept 2.1 The Atom Lesson Essential Question(s): What is an atom? How are the sub-atomic particles of an atom organized? What are some of the properties of the sub-atomic particles? Vocabulary: Atom, Nucleus, Proton, Neutron, Electron, Electron Cloud Goals Define atom. Describe the structure of the atom in terms of the nucleus (proton & neutron) and electron cloud (electron). Identify the charge, size, and locations of the proton, neutron, and electron Concept 2.2 The Periodic Table Lesson Essential Question(s): Vocabulary: How are the elements on the periodic table organized? How can the number of protons, electrons and neutrons be determined for a given atom using the Periodic Table? Periodic table, Period, Group, Family, Atomic number, Atomic Mass Goals Explain how elements are arranged in order of atomic number, usually in rows, so that elements with similar atomic structure (and hence similar chemical properties) appear in vertical columns. Define & describe how periods and groups are also used to organize the periodic table. Define atomic number & mass number. Determine the number of protons, electrons and neutrons for a given element using its atomic number and atomic mass. Concept 2.3 Elements Lesson Essential Question(s): Vocabulary: What is an element? How are electrons organized inside an atom? What are the three main types of elements? What are some properties metals, nonmetals, & metalloids? Element, Electron configuration, Valance electron, Lewis dot structure, Metal, Nonmetal, Metalloid Goals Define element. Introduce electron configuration Define valance electrons Draw Lewis Dot structures Classify elements as metals, nonmetals, or metalloids using the Periodic Table. List general properties of metal, nonmetal, and metalloid elements. Research & present specific properties about an individual element. Concept 2.4 Chemical Bonding Lesson Essential Question(s): What are chemical bonds and why do they form? List the different types of chemical bonds? What are the characteristics of covalent, ionic, and hydrogen bonding? Vocabulary: Chemical bond, Octet rule, Ionic bond, Covalent bond, Hydrogen Bond, Molecule, Ion Goals Define chemical bond and explain why they exist. State the octet rule and how it relates to reactivity. Describe ionic, covalent, and hydrogen bonds. Define molecule & ion.