PIB- Polyenes
advertisement

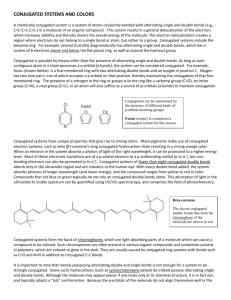
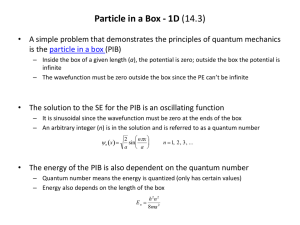
PIB and Highly Conjugated Molecules (14.7) • Conjugated molecules have alternating carbon-carbon single and double bonds – π-electrons can move freely along all carbon atoms in the conjugated portion of the molecule – The 1D PIB model can be crudely applied to the energy states of a polyene (e.g., βcarotene) • Energy levels (and wavefunctions) can be predicted given some information – Energy depends on quantum number, mass of particle (electron), and length of the box – Number of nodes in the wavefunction can be predicted based on the quantum number Electronic Spectroscopy of Highly Conjugated Molecules (14.7) • Highly conjugated molecules often have color associated with them – Absorb certain colors of visible light, so we see complimentary colors reflected back to us – Light is absorbed by an electron that is promoted from one state to a higher energy state – If PIB model holds, we can calculate the energy of the photon absorbed if we know two things: length of the box and the energy levels the electron starts in and ends in • Length of the box can be approximated by summing up the carbon-carbon bond lengths in the conjugated part of the molecule – Box is capped by double bonds, with single and double bonds alternating in the interior – For β-carotene, we have 10 C-C single bonds (0.154 nm each) and 11 C-C double bonds (0.133 nm each) • Energy levels are determined by number of double bonds in the system – Electron is promoted from last occupied π-bond (HOMO) to an unoccupied π-bond (LUMO) – For β-carotene: HOMO (n = 11) and LUMO (n = 12) Electronic Spectroscopy of Highly Conjugated Molecules (Con’t) • Energy of the photon absorbed can be predicted from formula for PIB energy levels – Knowing the quantum numbers involved simplifies the formula (watch units!) h hc E n 1 E n E n 1 E n 2 n 1 h 2 8 ma 2 • We can also predict what happens when we change some of the conditions of the problem – When conjugation is increased, the length of the box is increased (how does this affect energy of the electronic transition?) – If conjugation increases, what else changes?