Particle-in-a-box
advertisement
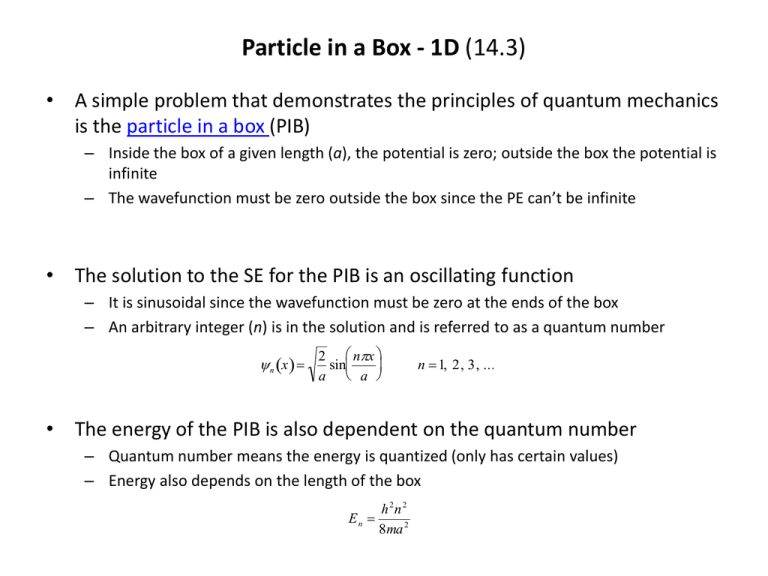
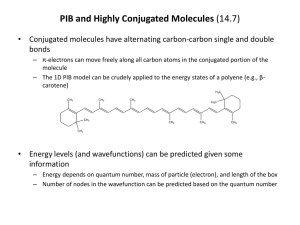
Particle in a Box - 1D (14.3) • A simple problem that demonstrates the principles of quantum mechanics is the particle in a box (PIB) – Inside the box of a given length (a), the potential is zero; outside the box the potential is infinite – The wavefunction must be zero outside the box since the PE can’t be infinite • The solution to the SE for the PIB is an oscillating function – It is sinusoidal since the wavefunction must be zero at the ends of the box – An arbitrary integer (n) is in the solution and is referred to as a quantum number n x 2 nx sin a a n 1, 2, 3, ... • The energy of the PIB is also dependent on the quantum number – Quantum number means the energy is quantized (only has certain values) – Energy also depends on the length of the box h 2n 2 En 8ma 2 Properties of PIB Wavefunctions (14.3-14.4) • Each solution of the PIB SE represents a state of the system – The lower the state (smaller n) the lower the energy of the system – First state is referred to as the ground state; higher energy states are called excited states • Some interesting features arise from the solution of the PIB SE – As the quantum number increases, so does the number of values of x for which the PIB wavefunction equals zero (nodes) – The PIB probability densities show a number of interesting characteristics (non-uniform probabilities, regions of zero probability within the box) • PIB for higher dimensions (2D, 3D) show very similar behavior – Wavefunction is a product of 1D PIB wavefunctions – Energy is a sum of 1D PIB energies – Some energy levels are degenerate (have same energies) n n x, y n x n x x y x y 2 h 2 n x2 n y E 8m a 2 b 2 Excited States and Spectroscopy (18.1-18.2) • In order for a system to go from one state to another, it must absorb or emit a quantized amount of energy (radiation) – Light is often used to promote particles to higher states (absorption) – Light is often emitted when excited states go back to lower lying states (emission) h E i1 E i i 1, 2, 3, ... • Spectroscopy is the use of light to probe states in matter – Type of light needed depends on the physical problem one is interested in (i.e., the potential energy function) – Selection rules dictate whether two states can be connected by shining light on it (depends on the nature of the wavefunctions of the two states) • Does PIB model any real physical phenomena? – The spectroscopy of highly conjugated molecules (e.g., polyenes) can be explained quite well by PIB model Particle in a Box PIB Wavefunctions PIB Probability Densities Absorption and Emission of Radiation