sp 2 systems: pi bonds, conjugation
advertisement

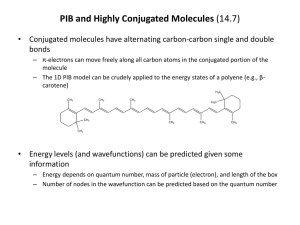
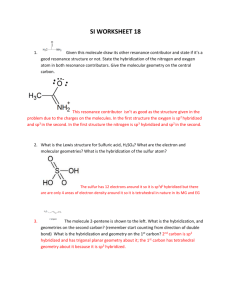
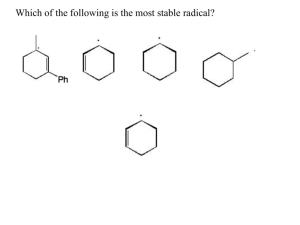
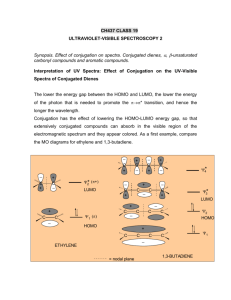
sp2 hybridized systems: pi bonds, conjugation, delocalization Major concepts sp2 hybridization is a planar geometry with a p orbital capable of overlapping to form pi bonds Conjugated systems are adjacent atoms containing pi bonds, lone pairs, and incomplete octets Atoms that are part of a conjugated system are sp2 hybridized To determine the hybridization of an atom, first look for conjugation, then consider VSEPR Conjugation allows for greater stability through delocalization of electrons, the physical picture of the resonance tool. Vocabulary Pi bond Free rotation Conjugated system Localized/delocalized electrons Students should be able to: Explain implications of pi bonds for bond strength, length, and free rotation Designate E/Z nomenclature Identify conjugated systems in a compound Determine the hybridization of any atom in a compound, taking into consideration conjugation Daily Problems 1. Why don’t we see this reaction occurring at room temperature? Explain in terms of the p orbitals. Z-but-2-ene E-but-2-ene 2. Which is the shortest carbon-carbon bond in trans-hex-3-en-1-yne? Explain. 3. Circle the atoms that are part of a conjugated system in these molecules and ions: 4. Designate these alkenes as E, Z, or neither. 5. Each of these nitrogen atoms is labeled with its correct hybridization. Explain how the hybridization was determined in each case. 6. Label the hybridization of each carbon, nitrogen, and oxygen atom in these molecules and ions, taking into account the concept of conjugation. Cumulative problems 7. Draw a resonance structure for the compound below, then draw its resonance hybrid. Where is their double bond character? Considering this resonance structure, why does it make sense to label the nitrogen as sp2 hybridized? 8. Indicate the conjugated system in each molecule, then draw significant resonance structures for each molecule. How does recognizing a conjugated system help you to draw resonance structures? How does drawing resonance structures help you determine which atoms are part of a conjugated system? Questions 9-13 refer to the Figure below: 9. The figure above shows a key reaction that takes place in the eye and allows for sight. The compounds are both retinal. One of them is called 11-cis retinal and the other is called all-trans retinal. Label them appropriately. 10. Circle the conjugated systems in both retinal molecules. 11. Retinal is made in the retina from Vitamin A, also called retinol. The only difference between retinal and Vitamin A is the change of one functional group. Consider the names, and then draw the structure of Vitamin A without referring to the internet or other source. 12. This reaction can only occur when a photon of light hits the eye. That photon of light has the exact amount of energy necessary to cause this reaction. Why is the energy needed to transform 11-cisretinal to all-trans-retinal? 13. When retinal is in your eye, it is chemically attached to a protein complex called rhodopsin. When retinal changes shape, it “pulls” on the protein rhodopsin, which causes effect on other proteins, which in turn leads to a chemical reaction, then electrical signal to be sent to your brain. Explain how light causes us to have perceptions to a non-science friend, using some of the terms and concepts from this unit. Extension problems 14. Here is a Lewis dot structure of benzene flat on the paper, a Lewis dot structure of benzene with the attempt to make it look like it is perpendicular to the paper (coming out at you), and finally an orbital overlap picture of the p-orbitals in benzene drawn sideways to the paper. Based on the picture, does it make more sense that each p orbital overlaps only one adjacent p orbital, or do you think that they all overlap both of their adjacent p orbitals?