UV-VISIBLE SPECTORMETER EXPERIMENT #2
advertisement

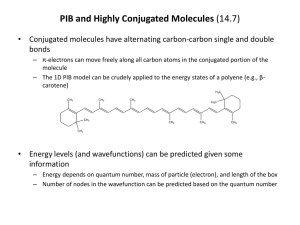
1 Advanced Chemistry – 2008 UV/Visible Spectroscopy Experiment #2 - Absorption Spectra of Conjugated Dyes Prepared by Dr. Dharshi Bopegedera 2 3 Using the Particle in a box model to investigate the Absorption Spectra of Conjugated Dyes The hypothesis in this experiment is that the electrons of a conjugated dye molecule (or any conjugated carbon-carbon bond system) can be modeled using the “particle in a box” model. We will make the following assumptions: All the carbon-carbon bonds in a conjugated system have equivalent bond lengths (1.5 bond length) Each carbon atom forms 3 sigma bonds. Each carbon atom contributes one valence electron to form a mobile electron cloud. This electron cloud moves along the carbon chain, above and below the plane of the chain. Potential energy of the electrons is constant along the chain. Potential energy of the electrons rises sharply to infinity at the ends of the chain. Theory: The above assumptions enable us to treat the electrons of a conjugated dye as a single free electron moving in a one-dimensional box of length L where L is the length of the conjugated carbon chain. -C=C-C=C-C=C-C=C-C=C-C=CA conjugated chain of length L (with 12 electrons) Potential Energy V Particle in a one-dimensional box of length L The quantum mechanical solution for the energy levels of an electron placed in a one-dimensional box of length L is: n2 h2 E whe re n 1, 2, 3, ...... n 8 m L2 lowest energy level is n=1 h = Planck’s constant m = mass of an electron L = length of the box 4 E n=5 E5 = 25h2/8mL2 n=4 E4 = 16h2/8mL2 LUMO lowest energy transition n=3 E3 = 9h2/8mL2 n=2 E2 = 4h2/8mL2 n=1 E1 = h2/8mL2 HOMO (Valence Electrons) Energy diagram for a conjugated carbon chain with 6 electrons (N = 6) Pauli exclusion principle limits the number of electrons in each level to 2. Therefore if there are N number of electrons, the ground state of the molecule will have N/2 number of levels completely filled (if N is an even number). In the above diagram N = 6 because we have drawn the diagram for a conjugated carbon chain with 6 electrons. When this conjugated system absorbs light, the valence electrons will be excited from the HOMO (n1 = N/2) to LUMO (n2 = N/2 + 1). The energy change for this transition is E E n - E 2 n 1 [N 2 1]2 h 2 8 m L2 [N - 2 ]2 h 2 8 m L2 [N 1] h 2 8 m L2 When this transition is excited with UV or visible light that has a frequency of and wavelength of : 5 E h 8 m L2 c h (N 1) hc [N 1] h 2 8 m L2 Equation A Equation A is very important because it enables us to test our hypothesis that the electrons of a conjugated system can be modeled with the particle in a box model. If this hypothesis is correct, the experimental value of L obtained using Equation A, and the theoretical value of L should be comparable. Compounds to be used in this experiment: 1, 4 diphenyl-1, 3-butadiene [Note: N = 4] 1, 6 diphenyl- 1,3,5-hexatriene [Note: N =6] 1, 8 diphenyl- 1,3,5,7-octatetraene [Note: N =8] Experiment: Prepare 1.0x10-6 M solutions (in 10 mL volumetric flasks) of each of the above dyes in cyclohexane solvent. Start from the stock solutions provided for you. Label clearly. Record the UV/visible spectra of the above three dyes. Determine the wavelength () of the lowest energy transition for each dye. These will be the HOMO to LUMO transitions. For these transitions, calculate the lengths of the boxes (L) using Equation A. These will be your experimental values for the lengths of the boxes. In order to obtain the theoretical lengths of the boxes, assume that each carbon-carbon bond has a length of 1.39 Å (0.139 nm). This is the carbon-carbon bond length in benzene. Use this value to calculate the theoretical lengths of the boxes in the above three compounds. Prepare a table so that the experimental and theoretical values can be easily compared. Calculate the % error of L for each compound. To this table, add the literature values (experimental values) from Reference 3. Use the experimental lengths of the boxes to determine the carbon-carbon bond length in the above compounds. Use the three values obtained from the three compounds to calculate the average carbon-carbon bond length. Compare your average value with 1.39 Å. Determine the % error. What would be a more appropriate bond length to use besides 1.39 Å? How does this value compare with your value of the carbon-carbon bond length? Comment on the validity of the hypothesis stated at the beginning of the experiment. References: 1. H. Kuhn, J. Chem. Phys., 1949,17, 1198. 2. C. W. Garland, J. W. Nibler and D. P. Shoemaker, Experiments in Physical Chemistry, Seventh Ed, McGraw Hill Publishers, New York, USA, (2003), p. 380-385 3. Bruce D. Anderson, J. Chem. Ed. 1997, 74, 985.