Quantitative Chemical Analysis 7e
advertisement

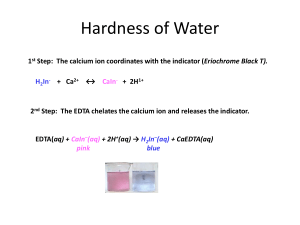
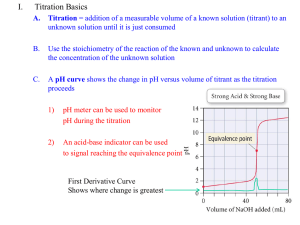
CHEMISTRY 59-320 ANALYTICAL CHEMISTRY Fall - 2010 Lecture 16 Chapter 12: EDTA Titrations 12-1 Metal-Chelate complexes • Metal ions are Lewis acids, accepting electrons pairs from electron-donating ligands that are Lewis bases. • Monodentate ligand: binds to a metal ion through only one atom. • Multidentate ligand: attaches to a metal ion through more than one ligand atom, also known as chelating ligand. • Chelate effect: the ability of multidentate ligands to form more stable metal complexes than those formed by similar monodentate ligands. • A titration based on complex formation is called a complexometric titration. • Following are structures of analytically useful chelating agents: 12-2 EDTA • EDTA is an abbreviation for ethylenediaminetetraacetic acid, a compound that forms strong 1:1 complexes with most metal ions. • EDTA is a hexaprotic system, designated H6Y2+. The highlighted, acidic hydrogen atoms are the ones that are lost upon metal-complex formation. • The neutral acid of EDTA is tetraprotic, with the formula H4Y. A commonly used reagent is the disodium salt, Na2H2Y · 2H2O • We can define α for each species as the fraction of EDTA in that form. For example, is defined above. Fractional composition diagram for EDTA • The equilibrium constant for the reaction of a metal with a ligand is called the formation constant, Kf, or the stability constant • The number is called the conditional formation constant, or the effective formation constant. It describes the formation of MYn−4 at any particular pH. Conditional formation constant • pH affects the titration of Ca2+ with EDTA. Below pH ≈ 8, the end point is not sharp enough to allow accurate determination. • The conditional formation constant for CaY2− is just too small for “complete” reaction at low pH. 12-3 EDTA Titration Curves • The titration curve is a graph of pM versus the volume of added EDTA. • The right side figure illustrates for reaction of 50.0 mL of 0.050 0 M Mn+ with 0.050 0 M EDTA, assuming Kf’= 1.15 × 1016, where the concentration of free Mn+ decreases as the titration proceeds. • There are three regions in an EDTA titration curve: (a) Before the equivalence point. (b) At the equivalence point. (c) After the equivalence point. • Since at equivalence point, there is exactly as much EDTA as metal in the solution, we can treat the solution as if it had been made by dissolving pure MYn−4. Some free Mn+ is generated by the slight dissociation of MYn−4: • The Ca2+ end point is more distinct than the Sr2+ end point because the conditional formation constant, for CaY2− is greater than that of SrY2− EDTA titration calculations • Problem 12-7: Consider the titration of 25.0 mL of 0.0200 M MnSO4 with 0.0100 M EDTA in a solution buffered to pH 8.00. Calculate pMn2+ at the following volumes of added EDTA and sketch the titration curve: (a) 0 ml, (b) 20.0 ml, (c) 40.0 ml, (d) 49.0 ml, (e) 49.9 ml, (f) 50.0 ml, (g) 50.1 ml, (h) 55.0 ml, (i) 60.0 ml. • Solution: from Table 12.2, we get log(Kf) = 13.89. At pH = 8.00, α(Y4-) = 4.2 x10-3. The above calculation will be carried out based on whether the titration is before or after the equivalence point. Here Ve*0.0100 M = 25.0*0.0200 M; Ve = 50.0 ml • (a) to (e) are before the equivalence point and will follow the same calculation equation: [Mn2+] * (0.025 + V) = 0.025 *0.02 – 0.01*V • (f) this is the equivalence point Kf’ = 4.2 x10-3 x 1013.89 = [MnY2-]/([Mn2+][EDTA]) = (0.02*0.025/0.075)/ ([Mn2+])2 [Mn2+] =8.179 x10-7. pMn2+ = • Calculations (g) to (i) are beyond the equivalence point, where [EDTA] can be obtained from the following [EDTA]*(0.025 + V) = 0.01*V – 0.02*0.025 then Kf’ = 4.2 x10-3 x 1013.89 = [MnY2-]/([Mn2+][EDTA]) A beaker containing 50.0 mL of 0.300 M Ca2+ at pH 9 is titrated with 0.150 M EDTA. The pCa at the equivalence point is: (a) 4.97. (b) 5.13. (c) 5.84. At the equivalent point, all Ca2+ ions have formed complex and free Ca2+ come from the dissociation of complex: CaY2Ca2+ + EDTA For Ca2+ Kf = 1010.65 ; α = 0.041 at pH = 9 2 CaY ' Kf 1.8 109 2 Ca EDTA 2 CaY 0.1 2 Ca 1.8 109 1.8 109 2 Ca 2 7.45 106 pCa= 5.13 12-5 Auxiliary Complexing Agents • It is a ligand that binds the metal strong enough to prevent metal hydroxide from precipitating, but weakly enough to give up the metal when EDTA is added. • Consider a metal ion that forms two complexes with the auxiliary agent. • The fraction of metal ion in the uncomplexed state M, can be calculated as • Ammonia complexes zinc in four different forms • EDTA titration in the presence of auxiliary complexing agent ammonia Example: EDTA titration in the presence of ammonia (b) When 50.0 ml EDTA is added, the system reaches equivalence point. (c) When 60.0 ml EDTA is added, the system is beyond equivalence point. Metal Ion Indicators • The most common technique to detect the end point in EDTA titrations is to use a metal ion indicator. • are compounds whose color changes when they bind to a metal ion. Useful indicators must bind metal less strongly than EDTA does. • Masking agent: In a direct titration, analyte is titrated with standard EDTA. The analyte is buffered to a pH at which the conditional formation constant for the metal-EDTA complex is large and the color of the free indicator is distinctly different from that of the metal-indicator complex. 12-7 EDTA titration techniques • • • • • Direct titration: Back titration: Displacement titration: Indirect titration: Masking agent:



![[MIn - ]/[HIn 2](http://s2.studylib.net/store/data/005622090_1-a61ecc244fc4a127a11c4475c40f111e-300x300.png)