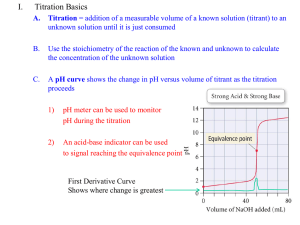
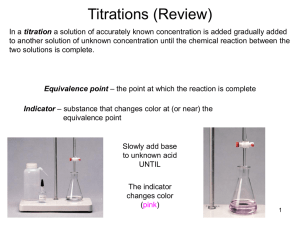
basic titration
advertisement
Basics of Titration by Michael Margreth Basics • One of the oldest analytical methods • Based on chemical reaction • Determination of the volume of a standard solution (titrant) • Standard solution contains a defined number of molecules • Measuring volume = counting molecules • TITRATION MEANS COUNTING! Titration Start Titrant Sample Titration Endpoint Titrant Titrant + Sample Titration End Titrant Titrant + Sample Principle of a Manual Titration Titrant - optical detection - manual control - manual addition Color indicator All in one! • Dosing • Measuring • Controlling • Evaluation Titrations Modes • Set Endpoint Titration SET • Monotonic Equivalence point Titration MET • Dynamic Equivalence point Titration DET • Karl Fischer Titration KFT Set Endpoint Titration Signal [pH/mV] volume Monotonic Equivalence Point Titration Signal [pH/mV] equivalence point volume Dynamic Equivalence Point Titration Signal [pH/mV] equivalence point volume Volume increment • • In MET mode the steps are always the same In DET mode the steps are calculated by the Titrando MET Mode DET Mode 800.00 pH pH 800.00 EP1 EP1 400.00 0.00 V [mL] 3.00 400.00 0.00 V [mL] 3.00 Karl Fischer Titration Signal [mg/min] volume Which mode for which titration? SET MET DET KFT defined endpoint slow reaction quick reaction Karl Fischer Titration non-aqueous titration duration has priority non S-shaped universal curve method (90%) Endpoint evaluation tangent method circle method derivative method Titration steps • sample preparation (homogeneity) • right electrode choice • possibly electrode conditioning • titrant preparation • titer determination • buret/ sample size choice • correct arrangement in titration vessel • stirrer rate • method parameters • results calculation • report Arrangement stirrer electrode buret Titer • What is a titer? Correction factor • Why do we need the titer? To know the exact concentration of the titrant • What is the unit? none • How is the titer determined? With titrimetric standards • When do I have to determine a titer? frequently Advantages • • • • • • • Absolute method Easy to carry out Carried out very rapidly Versatile method Highly reproducible and correct results Can be automated economical Hardware definition System Titrant definition Sensor definition Method definition Parameters Titration method Titration parameters Sensor Dosing device Stirrer Direct parameters Direct parameters Start conditions Titration parameters User defined parameters Measurement density • • 4 0 default high density • 9 low density DET Mode DET Mode 8,5 8,5 8,0 8,0 7,5 pH pH 7,5 EP1 7,0 EP1 7,0 6,5 6,5 6,0 6,0 0,0 0,5 1,0 1,5 2,0 2,5 3,0 V [ml] 3,5 4,0 4,5 5,0 5,5 6,0 -0,0 0,5 1,0 1,5 2,0 2,5 3,0 V [ml] 3,5 4,0 4,5 5,0 5,5 6,0 Stop conditions Evaluation Calculation Report Result report Curve report