Hardness of Water Presentation
advertisement
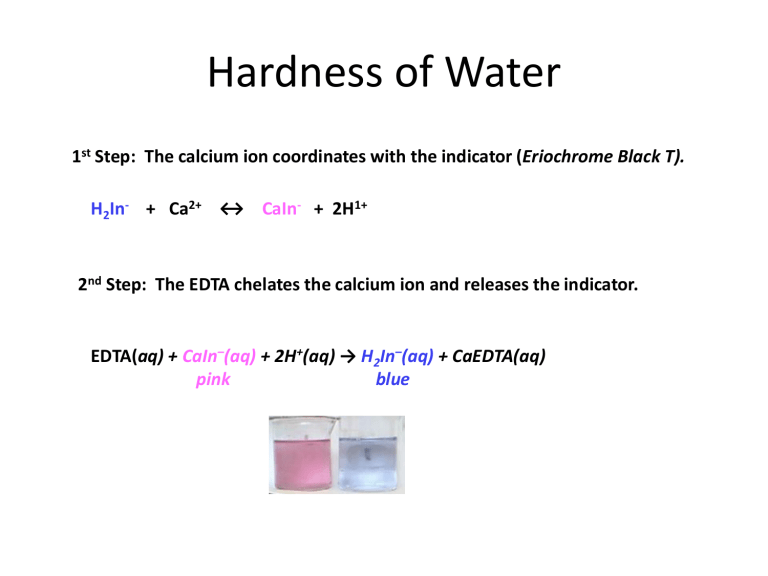
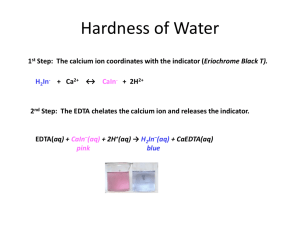
Hardness of Water 1st Step: The calcium ion coordinates with the indicator (Eriochrome Black T). H2In- + Ca2+ ↔ CaIn- + 2H1+ 2nd Step: The EDTA chelates the calcium ion and releases the indicator. EDTA(aq) + CaIn–(aq) + 2H+(aq) → H2In–(aq) + CaEDTA(aq) pink blue Sample Calculations: 1st Standardization of EDTA solution. EDTA(aq) + CaIn–(aq) + 2H+(aq) → H2In–(aq) + CaEDTA(aq) pink blue ? Mol/L 10.00 mL 38.00 mL It took 38.00 mL of EDTA solution to titrate 10.00 mL of standardized calcium ion stock solution (1.000 g CaCO3/L solution). Determine the molarity of the EDTA solution. (The molar mass of CaCO3 is 100.1 g/mol) 10.00 mL CaCO3 sol’n 10-3 38.00 x L EDTA sol’n 1.000g 1mol 1mol CaCO3 EDTA 100.1g 1mol CaCO3 sol ' n CaCO3 CaCO3 CaCO3 1000mL = 0.002629 mol EDTA L EDTA sol'n The mean (average) of your three trials will be used for your calculations. The mean (average) of your three trials will be used for your calculations. Example: Suppose the three calculated values of your standards were 0.002629, 0.002711, and 0.002649. Calculate the relative precision in parts per thousand (ppt). 1st find the mean: (0.002629 + 0.002711 + 0.002649)/3 = 0.002663 2nd: Find the absolute value of each trial’s deviation from the mean. absolute deviation for trial n: 𝑛 = |Na2EDTA]mean − [Na2EDTA]n| Trial 1: n = |0.002663 - 0.002629| = 0.000034 Trial 2: n = 0.000048 Trial 3: n = 0.000014 (0.000034+0.000048+0.000014) 3 x1000 12.02 ppt Estimated precision (ppt) 0.002663 Calculating Water Hardness (parts per million) Water hardness ppm ≈ mg CaCO3 ≈ 10-3g 1 L CaCO3soln 1000g = 10-6 Example: 25.00 mL of an unknown sample was titrated with 0.002663 M Na2EDTA solution. If it took 18.25 mL of 0.002663 M Na2EDTA solution to reach the endpoint, what was the “hardness of the water”? EDTA(aq) + CaIn–(aq) + 2H+(aq) → H2In–(aq) + CaEDTA(aq) pink blue 18.25 mL 25.00 mL 0.002663mol L EDTA 18.25 m L 0.002663 mol EDTA 1 mol CaCO3 100.1 g CaCO3 = 194.6 mgCaCO3 L EDTA 0.025 L CaCO3 sol’n or = 1 mol EDTA 1 mol CaCO3 LCaCO3 sol’n 18.25 mL Na 2 EDTA sol'n 0.002663mol Na 2 EDTA 1 mol CaCO3 100.1 g CaCO3 0.02500 L CaCO3 sol ' n L Na 2 EDTA sol'n 1 mol Na 2EDTA 1 mol CaCO3 Sample’s water hardness = 194.6 ppm Calculate your average hardness and your experimental precision from the three trials Eriochrome black T










![[MIn - ]/[HIn 2](http://s2.studylib.net/store/data/005622090_1-a61ecc244fc4a127a11c4475c40f111e-300x300.png)







